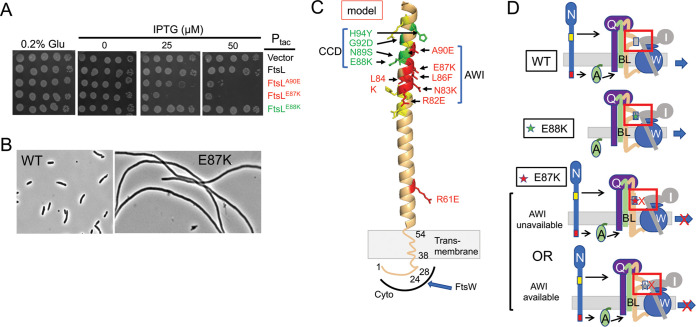
FIG 2.
Isolation of dominant negative mutations in ftsL. (A) Spot test of dominant negative mutations in ftsL. ftsL was subjected to random PCR mutagenesis, cloned downstream of the tac promoter in an expression vector containing an IPTG-inducible promoter (pJF118EH), and transformed into JS238. Transformants were screened for sensitivity to IPTG. ftsLWT and ftsLE88K (an activation allele) were included as controls and are not toxic. Several strong dominant negative mutations (ftsLE87K, ftsLL86F, and ftsLA90E) and two weak mutations (ftsLR61C and ftsLE24K) were obtained in this way (Table 1). Additional mutations were obtained by site-directed mutagenesis. (B) Dominant negative mutants inhibit division. Phase contrast micrographs of JS238 expressing ftsL or ftsLE87K (derivatives of pKTP100 [Ptac::ftsL]) grown in liquid culture and induced with 50 μM IPTG for 2 h. Induction of the other alleles also inhibited division (Table 1). (C) FtsL, residues 54 to 99, was modeled (for illustration purposes) as an alpha helix since it is thought to form a continuous alpha helix with the TM, and this region is also thought to form a coiled coil with FtsB. Altering the residues in green leads to activation mutations, whereas altering those residues in red results in dominant negative mutations. Altering the residues in yellow had no effect. Note that the activation mutations affect residues that lie mostly on one side of the helix, whereas the dominant negative mutations affect residues that lie mostly on the other side. The red residues (including L86 and E87) identify a region designated AWI (activation of FtsWI). The positions of residues 24 and 28 in the cytoplasmic domain are indicated along with the transmembrane (TM) domain. The cytoplasmic domain of FtsL is required to recruit FtsW, which in turn recruits FtsI. (D) Cartoons depicting the effect of various mutations on the activation of FtsWI according to the model. Top, FtsN action makes AWI available; middle, FtsLE88K is less dependent upon FtsN as the E88K substitution makes AWI available; bottom, FtsLE87K is resistant to FtsN action, and AWI does not become available or is defective in interaction with FtsWI.