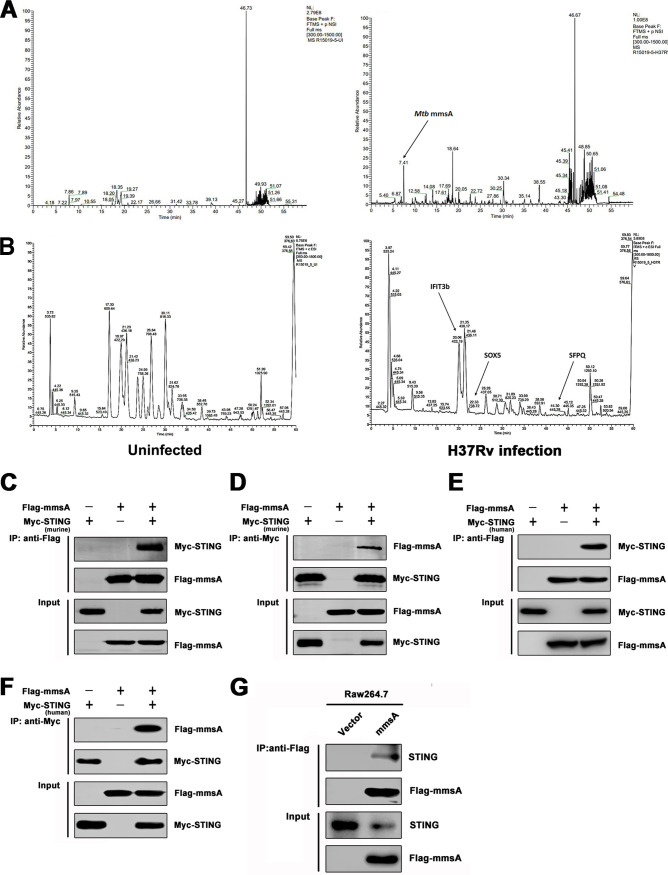
FIG 1.
M. tuberculosis MmsA is a binding partner of STING. RAW264.7 cells were infected with H37Rv for 6 h at an MOI of 10, and then the cell lysates were subjected to immunoprecipitation with anti-STING antibody or normal IgG. The immunoprecipitates were subjected to SDS-PAGE and identified by mass spectroscopy, which showed the mass spectroscopy spectrum of the STING protein complex searched against the mycobacterial proteome (A) and the mouse proteome (B). The arrows indicate the peaks of the candidate proteins interacting with STING as mycobacterial MmsA (A) and as murine IFIT3b, SOX5, and SFPQ (B) in H37Rv-infected (right) cells. HEK293T cells were transfected with vector or Flag-tag MmsA and murine Myc-tag STING for 48 h. Cell lysates were immunoprecipitated with the anti-Flag antibody (C) or anti-Myc antibody (D) and then immunoblotted with the indicated antibodies. HEK293T cells were transfected with vector or Flag-tag MmsA and human Myc-tag STING for 48 h. Cell lysates were immunoprecipitated with the anti-Flag antibody (E) or anti-Myc antibody (F) and then immunoblotted with the indicated antibodies. (G) The cell lysates from RAW264.7 cells stably expressing Flag-MmsA (RAW-MmsA) and RAW-Vector were immunoprecipitated with the anti-Flag antibody and analyzed by immunoblotting with the indicated antibodies.