Figure 2.
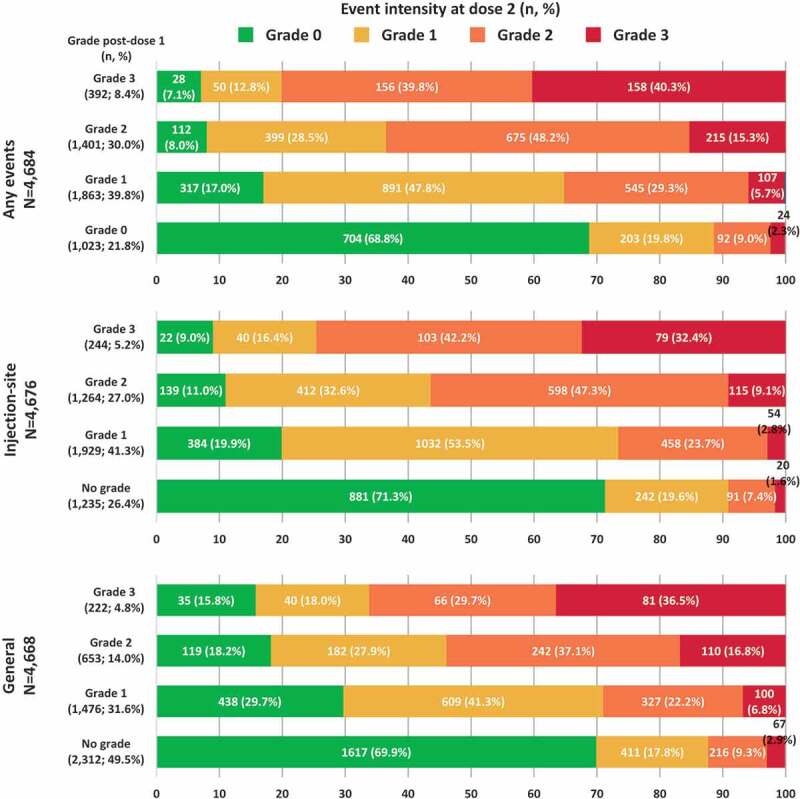
Intensity of any events, solicited injection site events and general events reported after dose 2 stratified by the intensity reported after dose 1 (TVC reactogenicity)
Footnote: TVC, total vaccinated cohort; N, number of participants with both doses administered having the corresponding grade at dose 1; n (%), number (percentage) of RZV vaccinees with events at a specific grade. Note: Injection site events included: pain at the injection site, redness at injection site and swelling at the injection site. General events included any solicited experiences which did not occur at the site of injection of RZV vaccine as: fatigue, gastrointestinal symptoms (nausea, vomiting, diarrhea and/or abdominal pain), headache, myalgia, shivering and fever. There were four injection site events and five general events with missing grading at dose 1 and 6 events (3 for each injection site and general) with missing grading at dose 2.
