FIGURE 3.
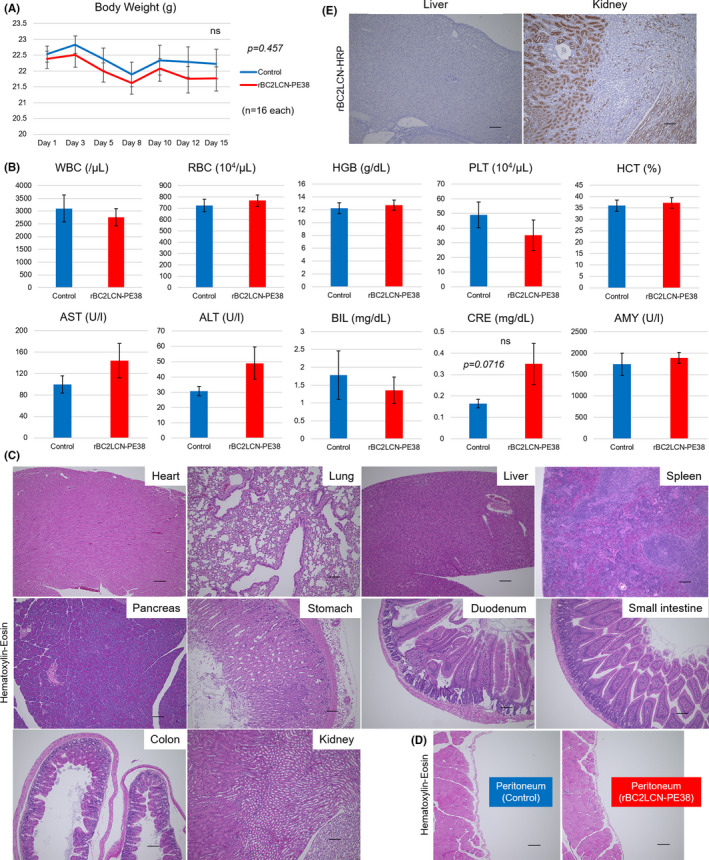
Evaluation of toxicity and adverse effects of rBC2LCN‐PE38 in in vivo mouse xenograft models. A, Body weight change during experimental period. B, Hematological findings on Day 15. The levels of 10 hematological examination parameters, including complete blood count (white blood cells [WBC], red blood cells [RBC], hemoglobin [HGB], platelets [PLT], and hematocrit [HCT]), aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (BIL), amylase (AMY), and creatinine (CRE) were measured. ns, not significant. C, Histological analysis of major organs, including heart, lung, liver, kidney, spleen, pancreas, stomach, duodenum, small intestine, and colon (scale bar: 100 µm). D, Histological analysis of the peritoneum. E, Histochemical staining for rBC2LCN in the major organs (scale bar: 100 µm)
