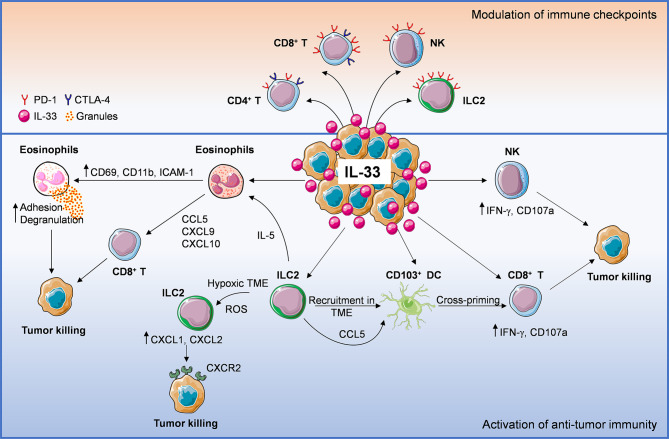
Figure 1.
Anti-tumoral mechanisms of interleukin-33 (IL-33) in the tumor microenvironment (TME). IL-33 administration or its physiological expression within the TME leads to direct or indirect recruitment of several immune effector cells such as eosinophils, ILC2, DC, NK cells, CD8+, and CD4+ T cells, establishing an immune cross-talk or directly controlling tumor growth. ILC2 cells can: 1) directly induce tumor cell killing through CXCL1/CXCL2 release and binding to tumoral CXCR2, 2) promote the recruitment of eosinophils via IL-5 production, 3) release CCL5 that facilitates CD103+ DC recruitment and cross-priming of CD8+ T cells. Following IL-33 exposure, eosinophil recruitment may result in: 1) direct tumor cell killing via adhesion-dependent degranulation and 2) release of CD8+ T cell-attracting chemokines (CCL5, CXCL9, CXCL10). Moreover, IL-33 can activate NK, CD8+ T (directly or via stimulation of cross-presenting DC) and CD4+ T cells, promoting anti-tumor effector responses. These events may be hindered by concomitant recruitment of ST2+ Treg cells. Lastly, IL-33 also up-regulates programmed cell death-1 (PD-1) on T lymphocytes (especially CD8+ T), NK cells and ILC2, as well as CTLA-4 on T cells, suggesting that this cytokine may improve the therapeutic response to immune checkpoint inhibitors.