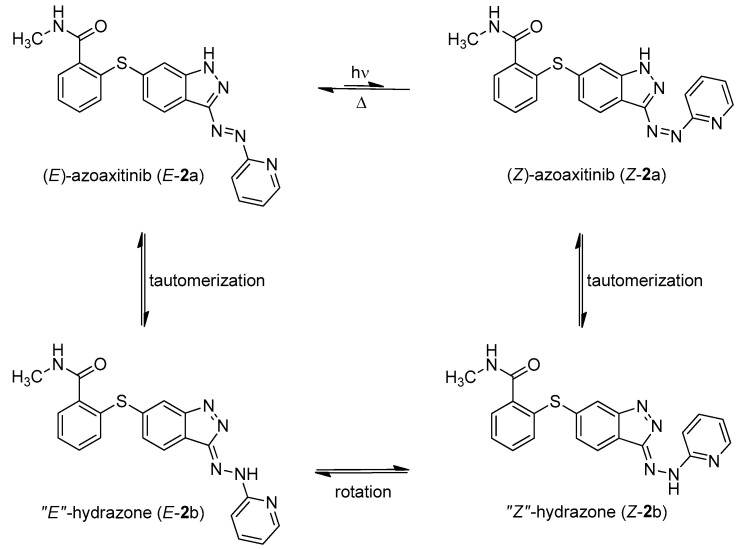
Figure 2.
Azo-hydrazone tautomerization of azoaxitinib (2). Due to the tautomeric equilibrium, the azo double bond is temporally turned into a free rotating single bond. This allows for fast relaxation from Z-isomer (Z-2a) to the thermodynamically stable E-isomer (E-2a) by rotation and subsequently back tautomerization.