Fig. 2. INS variants impair proinsulin oxidative folding and ER export.
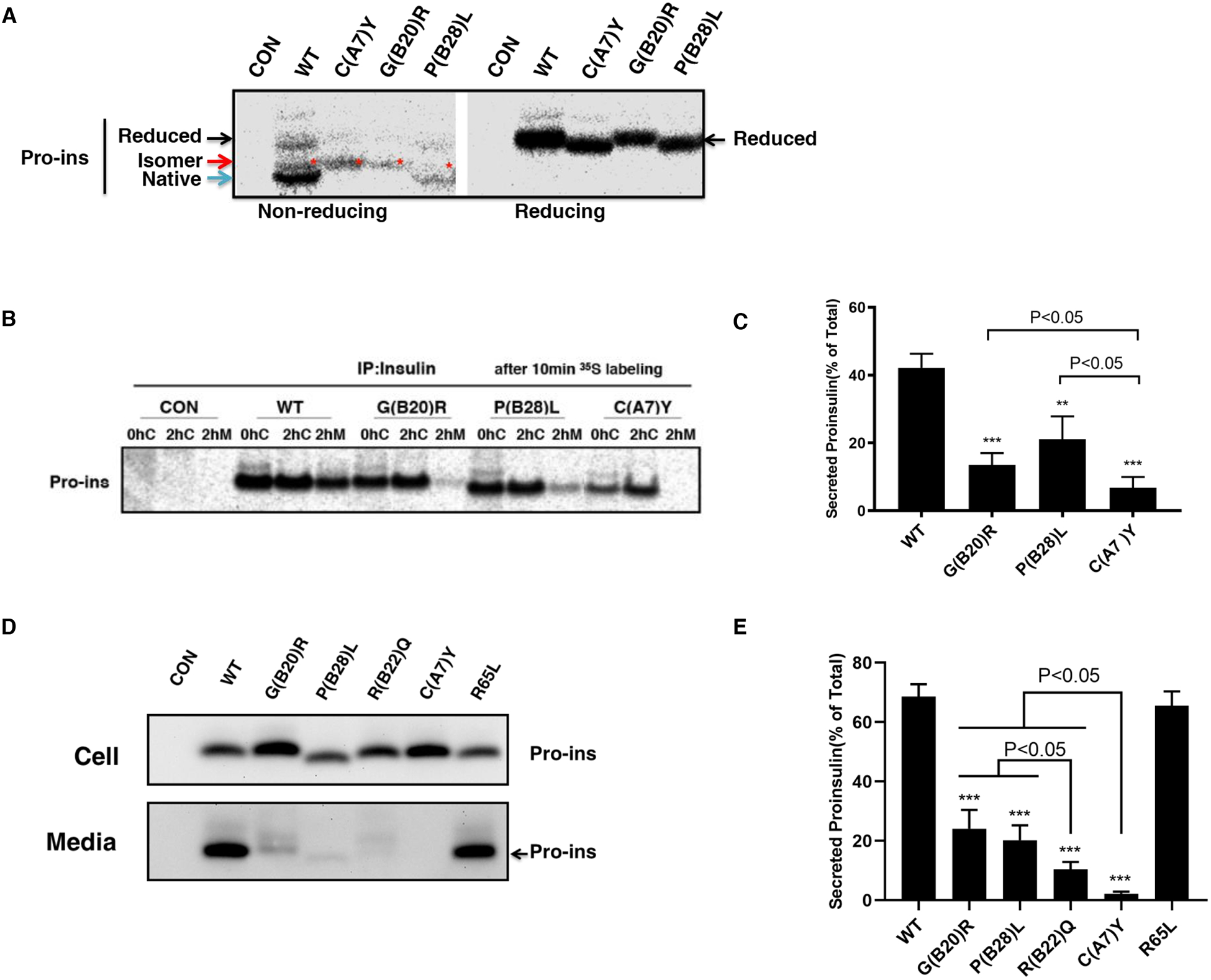
A. 293T cells were transfected with plasmids encoding wild-type (WT), or mutants C(A7)Y, G(B20)R, P(B28)L. At 48 h post-transfection, the cells were labeled with 35S-Met/Cys for 10 min. The newly synthesized proinsulin were precipitated with anti-insulin and analyzed by Tris–tricine–urea–SDS-PAGE under both non-reducing or reducing conditions. Reduced forms proinsulin marked by the black arrow, native forms marked by the blue arrow, disulfide isomers marked by the red arrow and star. B. 293T cells were transfected as Fig. 2A and pulse-labeled at 48 h with 35S-Met/Cys for 10 min followed by 0 or 2 h chase. Both cell lysates harvested after 0h (0hC) or 2h (2hC) chase and chase media (2hM) were immunoprecipitated with the anti-insulin and analyzed in 4–12% NuPage gel under reducing conditions with autoradiography. C. The secretion efficiency of WT or mutant proinsulin from at least three independent experiments shown in Fig. 2B was quantified using ImageJ. The results were shown as mean±SD, ** p <0.01 and *** p <0.001 comparing to WT (ANOVA test). D. 293T cells were transfected with plasmids encoding WT or mutant proinsulin as indicated. At 24 h post-transfection, the culture media were changed. After additional 24 hour incubation, the media were collected and cells were lysed. Both media and lysates were subjected to western blotting using anti-proinsulin antibody. E. The secretion efficiency of WT or mutant proinsulin under steady state from at least three independent experiments shown in Fig. 2D was quantified using ImageJ. The results were shown as mean±SD, *** p <0.001 comparing to WT (ANOVA test).
