Scheme 2.
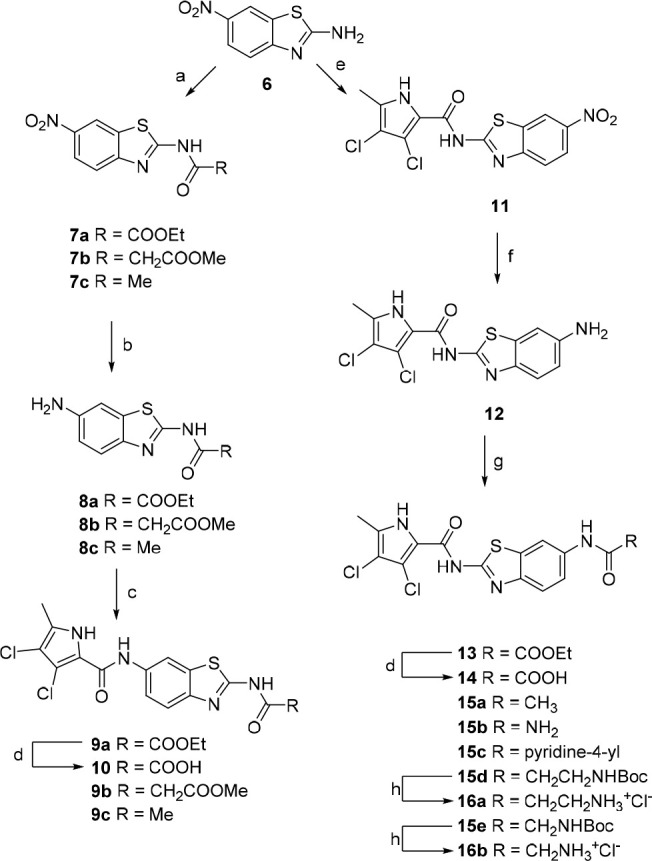
Reagents and conditions: (a) corresponding acyl chloride, Et3N, 1,4-dioxane, rt, 4 h; (b) H2, 10% Pd/C, EtOH (for 8a and 8c) or MeOH (for 8b), rt, 24 h; (c) 3,4-dichloro-5-methyl-1H-pyrrole-2-carbonyl chloride, pyridine, DCM; (d) 1 M NaOH, MeOH, rt, 24 h,; (e) 3,4-dichloro-5-methyl-1H-pyrrole-2-carbonyl chloride, toluene, reflux, 16 h; (f) SnCl2·2H2O, EtOH, reflux, 12 h; (g) ethyl oxalyl chloride, Et3N, 1,4-dioxane, rt, 8 h (for the synthesis of 13); (g) Ac2O, Et3N, DCM, rt, 2 h (for the synthesis of 15a); or CDI, DMF, rt, 3 h; NH3, rt, 16 h (for the synthesis of 15b); or nicotinic acid, EDC, NMM, HOBt, DMF, rt, 12 h (for the synthesis of 15c); or corresponding Boc-amino acid, EDC, NMM, HOBt, DMF, rt, 12 h (for the synthesis of 15d–e); (h) 4 M HCl, 1,4-dioxane, rt, 5 h.
