Scheme 1. General Synthetic Routes to Phosphinate Esters.
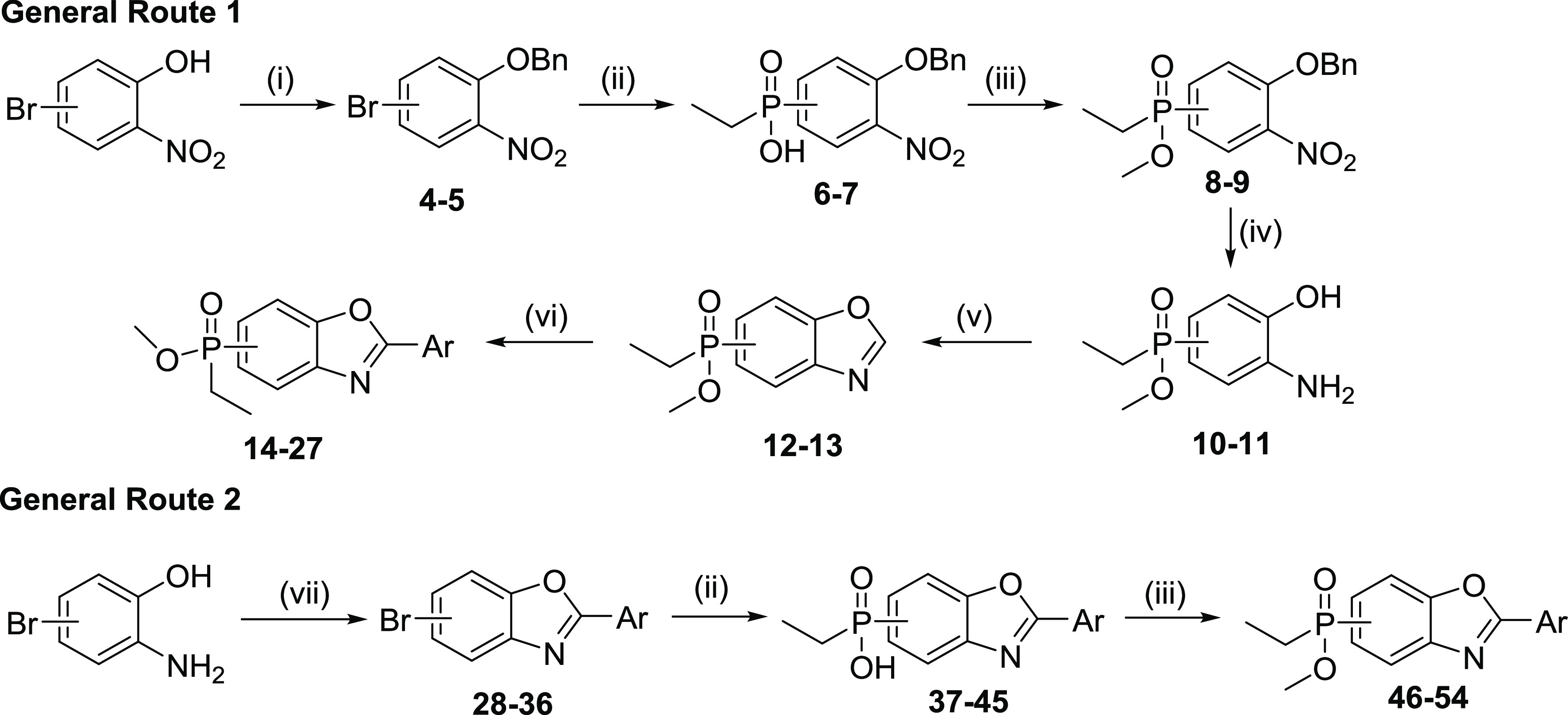
Reagents and conditions: (i) BnBr, K2CO3, acetone, 60 °C, 6 h, (yields 97% and 74% for 4 and 5, respectively); (ii) EtPH(O)OH, Pd(OAc)2, Xantphos, DIPEA, DME/toluene, 90 °C, 2 h, (yields 71–98%); (iii) (1) SOCl2, DMF, 70 °C, 1–2 h; (2) DIPEA, MeOH, DMAP, CH2Cl2, rt, 30 min (yields 41–85%) or MeOH, rt, 15 min for 9 (yield 81%); (iv) 10% Pd/C, EtOH/CH2Cl2, H2, rt, 3 h (yields 94–96%); (v) CH(OEt)3, PPTS, xylenes, 140 °C, 3 h (yields 91–95%); (vi) bromoaryl, Pd(PPh3)2Cl2 or Pd(dppf)Cl2, CuI, Cs2CO3, dioxane, 110 °C, 12 h (yields 7–40%) or bromoaryl, Pd(OAc)2, N-Xantphos, 1 M LiHMDS, DME, 85 °C, 1 h, for 14a, 15 (yields 22 and 12%, respectively) or chloroaryl, In(OTf)3, dioxane, 100 °C, 24 h, (yield 26%); (vii) (1) ArCOCl, xylenes, 155 °C, 1 h; (2) MsOH, 155 °C, 2–3 h (yields 12–81%).
