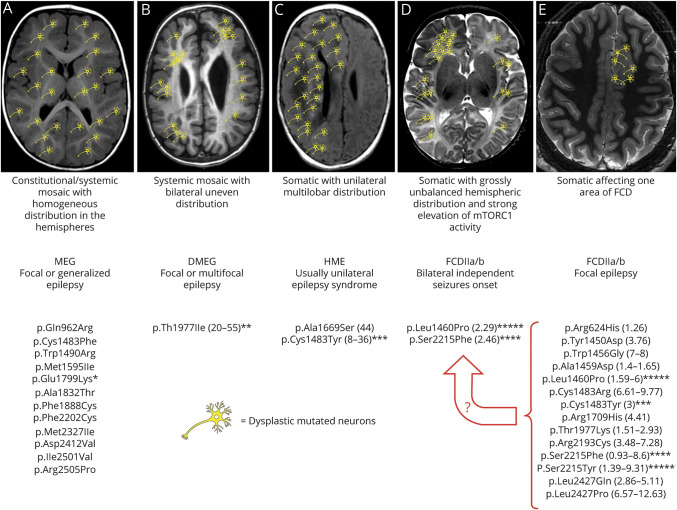
Figure 5. Schematic representation of the different possible combinations between specific MTOR mutations, mutant cells distribution, malformations of cortical development, and epileptogenesis.
Asterisks indicate mutations whose hyperactivation properties have been tested in parallel in functional studies; the number of asterisks reflects the level of MTOR hyperactivation observed.3 (A) Constitutional and systemic mosaic mutations resulting in homogeneous and widespread distribution of mutant cells in both hemispheres cause diffuse cortical malformations, with focal or generalized epilepsy of variable severity. These mutations are supposed, or have been demonstrated, to be moderately hyperactivating. (B) Systemic mosaic mutations may also cause mutant cells to be unevenly distributed in the brain hemispheres and cause a gross bilateral asymmetric malformation in which they are, however, present in both hemispheres. The only mutation associated with this phenotype has been functionally tested and has shown intermediate hyperactivating properties. (C) Somatic mutations with unilateral multilobar distribution cause hemimegalencephaly or large multilobar dysplasia and epilepsy, a syndrome which is usually unilateral. (D) Somatic mutations with strong mTORC1 activation, such as those identified in patients 1 and 2, have been associated with FCDII and intractable ipsilateral focal seizures at onset. Onset of contralateral seizures after the hemisphere harboring the FCD was removed strongly suggests a grossly unbalanced hemispheric distribution with foci of mutant cells being also present contralaterally and capable of generating seizures at some point. (E) Somatic mutations with low levels of mosaicism affecting 1 hemisphere cause FCDII and focal epilepsy as it was the case in (D) before hemispherectomy. These mutations tend to be highly hyperactivating, and if present at even very low AAF in the contralateral hemisphere might cause contralateral epileptogenesis at some point. Whether a critical mass of mutated cells or a certain level of aggregation also influences their epileptogenic potential remains unknown. A circumscribed distribution of MTOR mutations and dysplastic epileptogenic tissue as represented in (E) remains the most desirable eventuality because, as demonstrated by the need for progressively larger resections in patients 1 and 2 (the latter represented in (D)), even in (E) dysplastic cells might also be widely distributed within the hemisphere harboring the visible dysplasia and cause persisting-relapsing seizures after focal resection. Mutational data have been extracted from OMIM (omim.org/entry/601231) and HGMD professional (portal.biobase-international.com) databases. Numbers in parentheses represent the range of mosaicism percentage identified for each mutation. AAF = alternative allele fraction; DMEG = dysplastic megalencephaly; FCDII = focal cortical dysplasia type II; HME = hemimegalencephaly; MEG = megalencephaly; mTOR = mammalian target of rapamycin.