Figure 3. Combinatorial in vivo effects of BMJ and GEM against growth of PanC-PDX tumor explants.
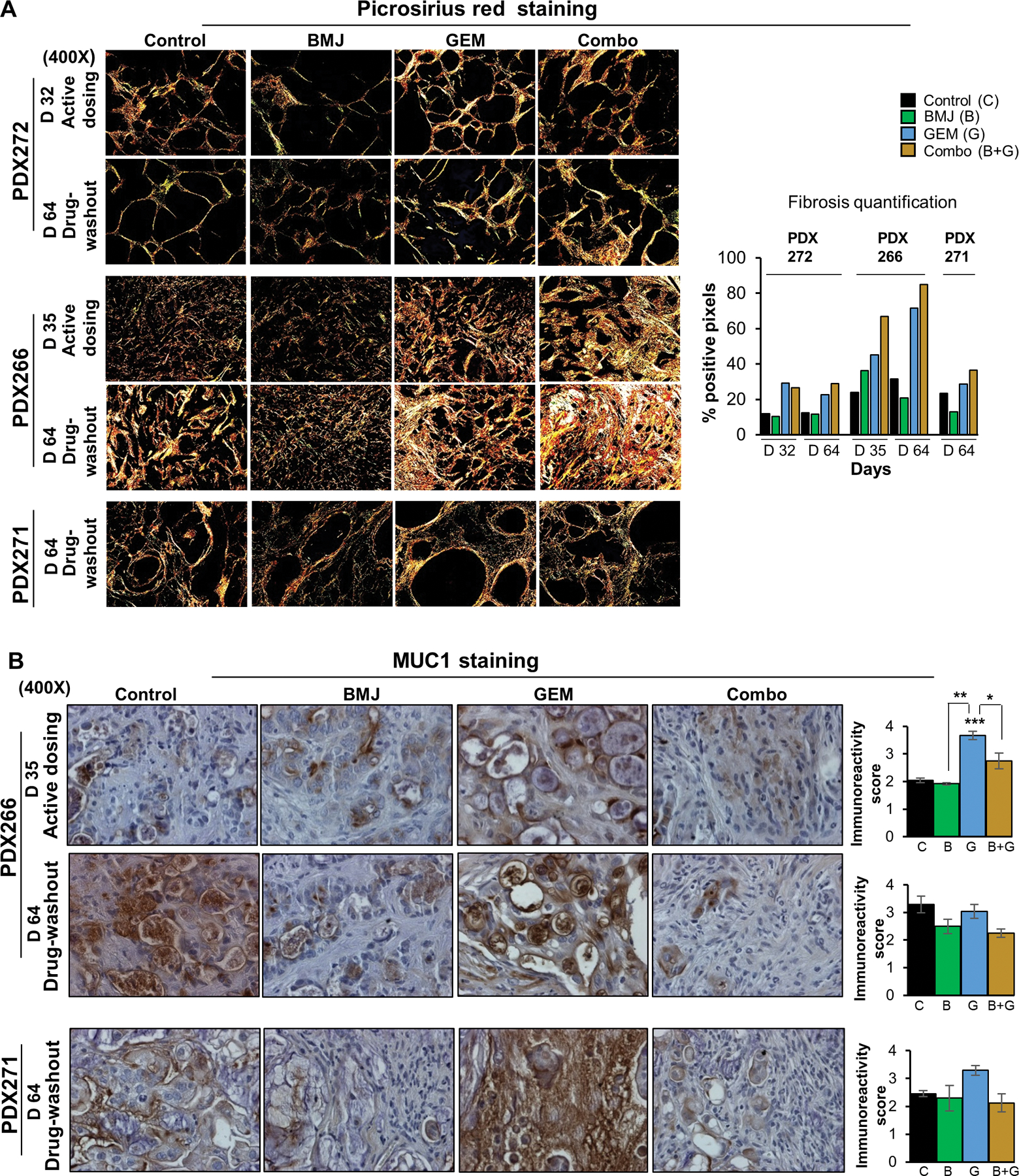
Right and left panel depict longitudinal assessment of tumor volumes (mm3) as a function of time (measured biweekly). (A-C, left panel) depicts tumor volumes during active dosing with drugs (either alone or as combination ‘Combo’)-until day 32 for both PDX272 and PDX271, and day 35 for PDX266 tumors; (A-C, right panel) depicts tumor volumes during drug-washout phase (tumor regrowth was measured post-treatment termination until day 64). Values are expressed as mean ± S.E.M. Tumor trends: black (untreated controls) green (BMJ-exposed), blue (GEM-exposed), and yellow (Combo-exposed) groups. Drug treatments were initiated at day 1: control (untreated); BMJ oral gavage in water (200mg/kg body wt.; 5 days a week); GEM (i.p. 50mg/kg biweekly in saline); and BMJ and GEM combination (Combo). No. of explants per group in active dosing phase: PDX272 [control (n=12), BMJ (n=11), GEM (n=12), Combo (n=12)]; PDX266 [control (n=10), BMJ (n=9), GEM (n=10), Combo (n=11)] and PDX271 [control (n=5), BMJ (n=5), GEM (n=4), Combo (n=4). (D) Comparison of PDX-tumor volumes (fold change) at the start of drug-washout phase [(day 32 or day 35) represented by solid fill bars] to the tumor volumes at the end of drug-washout phase [day 64 represented by no fill bars]. No. of explants per group evaluated in drug-wash out phase: PDX272 [control (n=7), BMJ (n=3), GEM (n=6), Combo (n=6)]; PDX266 [control (n=4), BMJ (n=5), GEM (n=5), Combo (n=5)] and PDX266 [control (n=5), BMJ (n=5), GEM (n=4), Combo (n=4)]. ***p≤0.001, **p≤0.01 and *p≤0.05.
