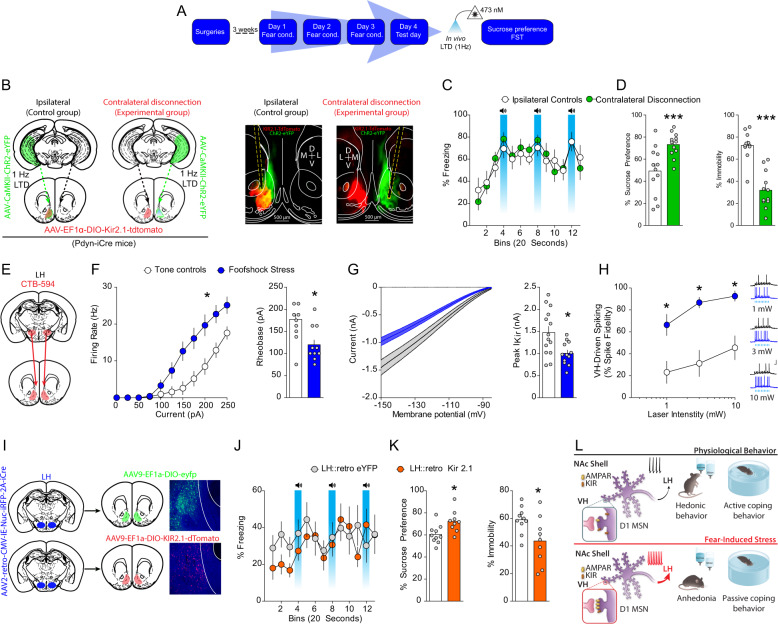
Fig. 8. Cooperation of synaptic and intrinsic plasticity in a disynaptic circuit drives stress-induced anhedonia and passive coping.
a Experimental timeline. b Mice were injected with unilateral AAV1-CaMKII-ChR2-eYFP into the VH. Contralateral disconnection and ipsilateral control group mice were injected with AAV1-EF1α-DIO-KIR2.1-2A-tdtomato into the contralateral or ipsilateral NAc, respectively. c Freezing on day 4 was not significantly different between control and footshock stress mice (n = 11–12 per group; two-way ANOVA; treatment main effect; F(1,21) = 0.01, p = 0.91; time main effect; F(14,294) = 8.83, p < 0.001; treatment × time interaction; F(14, 294) = 0.94, p = 0.52). d Contralateral disconnection of increased VH synaptic strength in the NAc and increased excitability of NAc D1-MSNs restores sucrose preference (n = 12; t-test; t(22) = 3.36, p = 0.003) and mobility in the forced swim test (n = 11–12; t-test; t(21) = 6.42, p < 0.0001). e CTB-594 was injected into the LH of WT mice. LH-projecting D1-MSNs were recorded. f Input–output curves and rheobase of action potential firing in LH-projecting NAc D1-MSNs. Input–output curves (n = 12–13 per group; two-way ANOVA; treatment × current interaction; F(10, 230) = 8.92, p < 0.0001). Rheobase (t-test; t(23) = 3.77, p = 0.001). g Decreased IRK currents in LH-projecting D1-MSNs from stressed mice (t-test; t(24) = 2.84, p = 0.0091). h AAV1-CaMKII-ChR2-eYFP and CTB-594 were injected into the VH and LH, respectively, of mice. LH-projecting NAc D1-MSNs were recorded. Increased input–output transformations in the VH-NAc D1-MSN-LH pathway in naïve and footshock stress mice (two-way ANOVA; treatment main effect; F(1, 13) = 29.84, p = 0.0001; stimulation intensity main effect; F(2, 26) = 16.11, p < 0.0001; treatment × stimulation intensity interaction; F(2, 26) = 1.53, p = 0.236). i AAV2-retro-CMV-iRFP-2A-Cre was injected into the LH, while AAV9-EF1a-DIO-KIR2.1-2A-tdtomato or AAV9-EF1a-DIO-eYFP was injected into the NAc of WT mice. j Freezing on day 4 in mice expressing control fluorophore or KIR2.1-tdtomato in LH-projecting D1-MSNs. k Overexpression of KIR2.1 in LH-projecting D1-MSNs rescues footshock stress-induced decreases in sucrose preference (n = 9–10; t-test; t(17) = 2.80, p = 0.012) and increases in immobility in the forced swim test (n = 9–10; t-test; t(17) = 2.417, p = 0.027). l Model depicting that increased information flow in a VH to NAc to LH circuit drives passive coping and anhedonia. This is mediated by increased synaptic strength of VH inputs to D1-MSNs, via increased AMPAR function and or number, and increased excitability, via decreased IRK function and/or number, in LH.