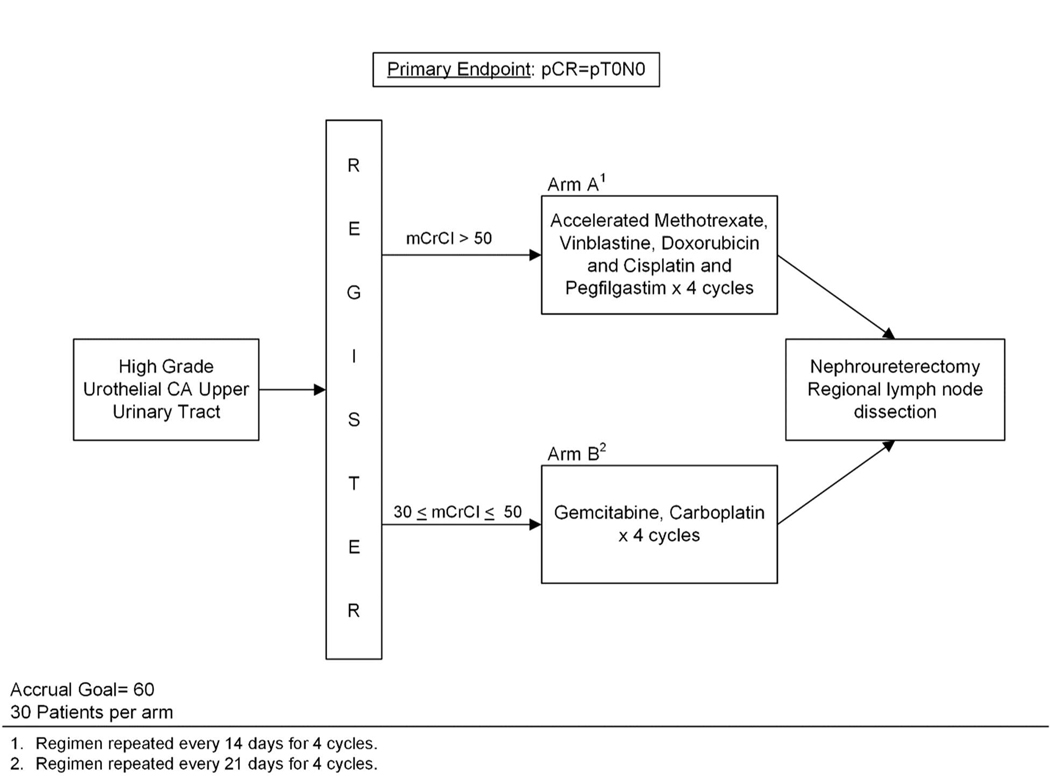
Figure 1.
Study schema. Accrual goal for this two-arm phase II trial was 60 patients (30 per arm) with high-grade upper tract urothelial carcinoma (HG UTUC). Patients were assigned to one of two treatment arms depending on their baseline creatinine clearance (CrCl). Primary endpoint was complete pathologic response (pCR), defined as pT0N0.