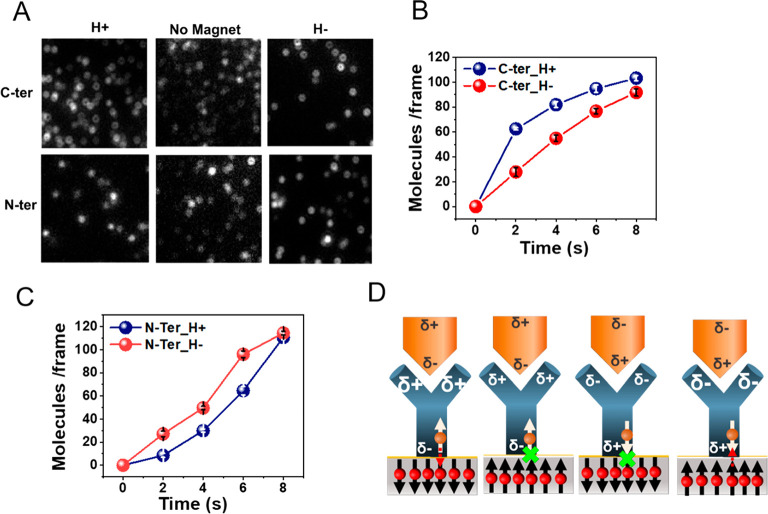
Figure 2.
Effect of surface magnetization on the kinetics of antigen–antibody binding. (A) Fluorescence microscope images of individual complexes formed between adsorbed anti-His antibodies and ClpB molecules His-tagged either at the C terminus (C-ter) or at the N terminus (N-ter). The interaction time is 2 s, with the North magnetic pole of the magnetized substrate pointing either UP (H+) or DOWN (H−) and also without the magnet as a control. For the C-terminal His-tag the number of adsorbed antigen molecules is larger for H+ while for the N-terminal His-tag the number is larger for H–. The number of molecules is calculated as described in the Methods section (Figure S3). (B) Reaction kinetics of the antibody with C-terminal His-tagged ClpB under the two magnetic orientations. (C) Reaction kinetics of the antibody with N-terminal His-tagged ClpB under the two magnetic orientations. (D) Schematic representation of the mechanism of the effect of spin on the antigen–antibody interaction. When the spin on the ferromagnet is pointing opposite to the momentary spin of the charge at the interface of the antibody and the ferromagnetic, charge flows more efficiently between the antibody and the surface. The charge flow facilitates charge redistribution in the antibody, which in turn increases the antigen–antibody binding rate.