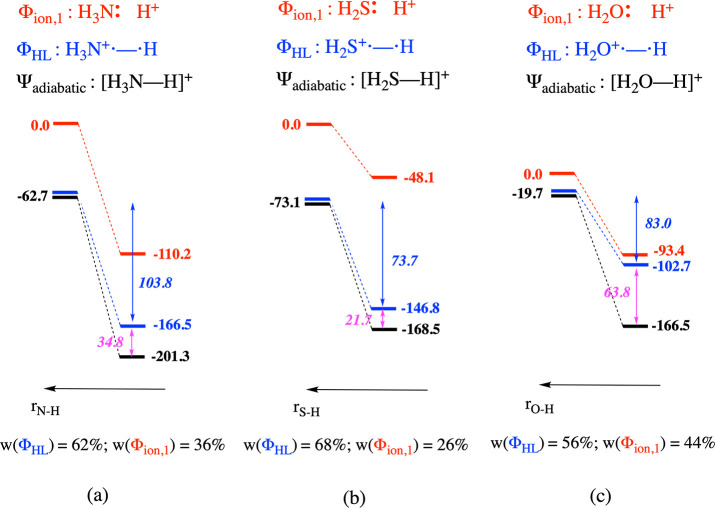
Figure 3.
Schematic representation of the bonding interaction and the evolution of the energy of the individual diabatic structures and the global adiabatic state associated with this process for (a) [H3N–H]+, (b) [H2S–H]+, and (c) [H2O–H]+. Note that the curve associated with Φion,2 is not depicted in this figure due to the significant energy gap between this curve and those associated with Φion,1 and ΦHL, respectively. Spin-pairing stabilization energies associated with ΦHL upon bond formation are depicted in italics in blue; resonance energies are shown in magenta. Weights of the individual structures in the adiabatic wave function at the optimal bonding distance are shown at the bottom of each panel.