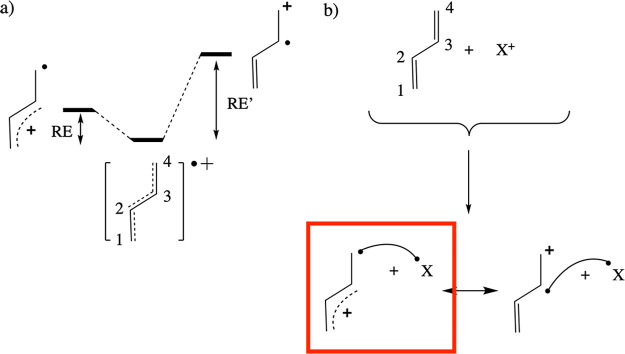
Figure 4.
(a) Radical cation species obtained upon oxidation of the neutral molecule R (here R = butadiene) is a mixture of several (semi)localized/diabatic structures; two such structures are shown for the example system: one in which the radical electron is localized on a terminal carbon (site 4), and one in which the radical electron is localized on a nonterminal carbon (site 3). To each of these localized structures, one can associate a resonance energy (RE), i.e., the amount of energy separating the full, delocalized species from the considered localized structure. (b) When a reaction partner X approaches molecule R and binds to it, the HL structure R+•–•X localizes, i.e., the radical electron in R+• has to end up at a specific site. The energetic cost of this localization process, inherently connected to this reaction, amounts to the corresponding resonance energy (the delocalization energy lost). The lower this RE “penalty” associated with a specific site, the more favorable the spin-pairing interaction will be when the X+ binds at this site. For the considered butadiene example (panel a), RE < RE′ (and the intrinsic spin-pairing stabilization is equal), so that the radical electron will preferentially localize on the extremal C sites. Consequently, the HL structure for C4–X bond formation can be expected to be lower in energy than the HL structure for C3–X bond formation.