Figure 2. Hh signaling paradigms during development.
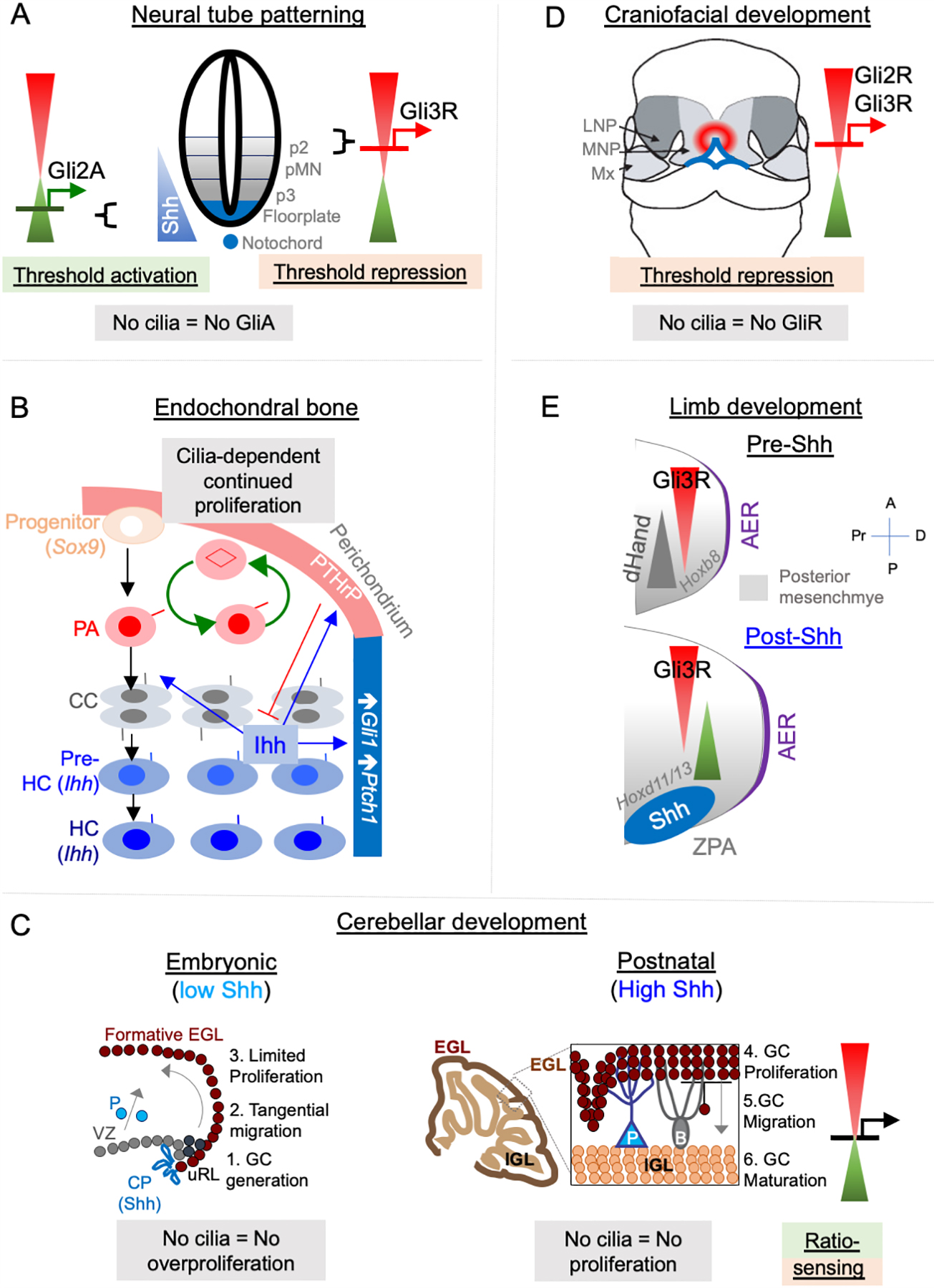
(A) Neural tube patterning. Shh is expressed from the notochord (blue). Gli2A-mediated threshold activation mediates floorplate and ventral most progenitor patterning. Lack of cilia prevents patterning of all ventral progenitors. Gli3R regulates intermediate-level patterning (not shown).
(B) Endochondral bone development. Chondrogenic progenitors differentiate into periarticular chondrocytes (PA), which further differentiate into columnar chondrocytes (CC), followed by forming prehypertrophic chondrocytes (Pre-HC) and hypertrophic chondrocytes (HC), both of which secrete Ihh. All these chondrocytes are ciliated. Ihh increases Gli1 and Ptch1 levels in adjacent perichondrium. Ihh also results in production of parathyroid hormone-like peptide (PTHrP) in periarticular cartilage, which prevents differentiation of CC to pre-HC in a negative-feedback loop. Lack of Gpr161 causes persistent slow proliferation of PA and prevents differentiation of PA into CC. Cilia disruption prevents continued proliferation from lack of Gpr161.
(C) Cerebellar development.
Left, Embryonic development (E15-E18). At this stage, Shh is expressed by choroid plexus (CP), which causes activation in adjacent ventricular zone (VZ). Purkinje neurons (P) are still translocating. Granule cell (GC) progenitors are generated in upper Rhombic lip (uRL), and tangentially migrate to the formative external granule layer (EGL). Proliferation in formative EGL is not affected by loss of cilia (unless overproliferation occurs from derepression arising from Gpr161 loss), and proceeds in the absence of Shh secretion by Purkinje neurons.
Right, Postnatal development (P0-P14). At this stage, Shh secreted by Purkinje neurons (P) causes proliferation of GC progenitors in EGL. Proliferation at this stage requires cilia. After GCs stop proliferating, they migrate radially on Bergmann glia (B) to form the internal granule layer (IGL). Proliferation at this stage can be affected by both lack of GliA and expression of GliR, suggesting sensing of GliA to GliR ratio by the GC progenitors.
(D) Craniofacial development. Threshold repression by both Gli2R and Gli3R prevents midfacial widening. Lack of cilia, or lack of both Gli2/3 causes mid facial widening, which is prevented by forced Gli3R expression. LNP, latera nasal process; MNP, medial nasal process, Mx, maxillary process.
(E) Limb development.
Pre-Shh stage, top. (E9.25-E9.75). Gli3R gradient is set up by posterior dHand gradient that is established in posterior mesenchyme.
Post-Shh stage, bottom. (E9.75 onwards). Shh expression from ZPA establishes posterior gradient of pathway targets such as Ptch1/Gli1. Anterior Gli3R gradient also regulates expression of genes in posterior mesenchyme such as Hoxd11/13. Lack of cilia causes decreased expression of Shh pathway targets but can cause preaxial polydactyly from increased 5’Hoxd gene expression arising from lack of Gli3R. Abbreviations: AER, anterior ectodermal ridge; ZPA, zone of polarizing activity; A, anterior; P, posterior, Pr, proximal; D, distal.
