Abstract
Objective:
Cerebral malaria (CM) affects 500,000 million children annually, 10% whom develop epilepsy within two years. Acute identification of biomarkers for post-CM epilepsy would allow for follow-up of the highest risk populations in resource-limited regions. We investigated the utility of EEG and clinical metrics obtained during acute CM infection for predicting epilepsy.
Methods:
We analyzed 70 EEGs recorded within 24 hours of admission for CM hospitalization obtained during the Blantyre Malaria Project Epilepsy Study (2005–2007), a prospective cohort study of pediatric CM survivors. While all studies underwent spectral analyses for comparisons of mean power band frequencies, a subset of EEGs from the 10 subjects who developed epilepsy and 10 age- and sex-matched controls underwent conventional visual analysis. Findings were tested for relationships to epilepsy outcomes.
Results:
Ten of the 70 subjects developed epilepsy. There were no significant differences between groups that were analyzed via visual EEG review; however, spectral EEG analyses revealed a significantly higher gamma-delta power ratio in CM survivors who developed epilepsy (0.23 ± 0.10) than in those who did not (0.16 ± 0.06), p = 0.003. Excluding potential confounders, multivariable logistic-regression analyses found relative gamma power (p = 0.003) and maximum temperature during admission (p = 0.03) significant and independent predictors of post-CM epilepsy, with area under receiver operating characteristics (AUROC) curve of 0.854.
Conclusions:
We found that clinical and EEG metrics acquired during acute CM presentation confer risk of post-CM epilepsy. Further studies are required to investigate the utility of gamma activity as a potential biomarker of epileptogenesis and study this process over time. Additionally, resource limitations currently prevent follow-up of all CM cases to surveil for epilepsy, and identification of acute biomarkers in this population would offer the opportunity to allocate resources more efficiently.
Keywords: Cerebral malaria, EEG, Epilepsy, Pediatric, Biomarkers
I. Introduction
Epilepsy affects 45–65 million people globally[1–3]. Acquired epilepsy as a result of brain injury is a significant contributor to this high global burden of disease, particularly in resource-limited regions where brain insults such as perinatal injury, traumatic brain injury, or central nervous system infections are common[4]. Identification of biomarkers for epileptogenesis would help identify those at highest risk of developing epilepsy, and potentially provide a pathway to alter the course from injury to epilepsy. Biomarkers for epileptogenesis have been investigated across a variety of data sources, including serological, radiographic, and neurophysiologic, with hope of identifying future risks of generating epileptic seizures, but results remain limited to date[5].
Unfortunately, the highly heterogenous nature of epilepsy, from etiology to manifestation, has constrained the search for biomarkers of epileptogenesis[6]. While certain brain insults can result in epilepsy, only a small proportion of the affected population develops the condition, and the time course in which this occurs is highly variable[7]. Thus, a pressing challenge is identifying a prevalent condition with a fairly homogenous mechanism of insult, high rate of occurrence, and high risk of subsequent epilepsy within a short time frame. Cerebral malaria (CM) is a unique condition fitting these criteria.
Annually, over half a million children develop acute cerebral malarial infections, with mortality rates up to 20%[8, 9]. CM is clinically defined as unarousable coma (persisting for at least 30 minutes after any seizure) and parasitemia in absence of other coma etiology[10]. Among survivors, one-third develop cognitive or behavioral symptoms over time and 10–16% develop epilepsy within two years of acute illness[11–14]. Pathophysiology of brain injury in cerebral malaria is consistent with the molecular mechanisms implicated in other forms of epileptogenesis, including inflammation, oxidative stress, and disruption of the blood-brain barrier[15, 16]. These pathophysiologic mechanisms may be plausible therapeutic targets, and thus raise prospects for productive epileptogenesis research in CM.
Published reports indicate a positive correlation between high fever and acute seizures during CM presentation with likelihood of post-malaria epilepsy[11], suggesting that clinical metrics contain information enabling conferring of post-CM epilepsy risk. However, other measures such as those obtained by electroencephalogram (EEG), may improve post-CM epilepsy risk estimates, but have not been extensively studied. Digital EEG is widely available in most urban-based tertiary hospitals in low- and middle-income countries where children with CM are most commonly managed due to the complexity and high mortality and morbidity risk of the condition[17]. Yet studies of EEG in CM have to date been by visual inspection, and have not revealed any relationships between conventional EEG findings (e.g. interictal abnormalities, background slowing) and likelihood of post-CM epilepsy[11, 18, 19]. Accordingly, knowledge if digital EEG analysis, specifically spectral analysis of relative power in specific EEG frequency, can improve post-CM epilepsy prediction remains unknown.
Thus, while CM has strong potential as a model for acquired epilepsy and epileptogenesis, investigations using EEG metrics as predictive markers of post-malaria epilepsy have been limited, and EEG remains an untapped source with high potential for predicting epilepsy in survivors. The range of measures that can be obtained by EEG can be coarsely divided into those derived from EEG analysis by visual inspection and those derived from quantitative, computer-based EEG analysis. In particular, quantitative analysis of the EEG frequency content from standard digital EEG recordings can provide, in humans, measures of power in the gamma frequency band reflecting the activity of fast-spiking GABAergic inhibitory interneurons that are predictably lost during epileptogenesis[20–26]. Relevant to the setting of low middle-income countries, where CM is prevalent, the capacity to extract EEG frequency content by fast Fourier transform (FFT) is widely available, and requires only a standard operating system and minimal computing power, which is already available to record the EEG[27]. Thus, given the minimal technical requirements, spectral EEG analysis is a feasible source of CM-induced epileptogenesis biomarkers, even in resource-limited regions.
2. Materials and Methods
2.1. Study design and participants
EEGs in this study were obtained with permission from the Blantyre Malaria Project Epilepsy Study (BMPES) database[11]. The original study design was a prospective cohort conducted at Queen Elizabeth Central Hospital in Blantyre, Malawi, from 2005–2007, during malaria season. From the original BMPES cohort, admission EEGs from 70 study children were available. For purposes of this study, the first recorded EEG from each patient was included in the analyses.
EEGs were recorded from patients admitted with CM within 24 hours of admission and daily until recovery of coma. CM was defined by clinical criteria including Blantyre coma scale ≤ 2 (a standardized scale of 0–5 for motor, verbal, and eye function used for assessing coma in CM [28, 29]) and the presence of malaria retinopathy. Recordings were made by a BioLogic CEEGgraph digital machine (Natus Medical Inc.) with a modified 10–20 system, meeting the American Electroencephalography Society guidelines for EEG recording[30]. EEG data were stored securely and de-identified before analysis. In the original study, acute EEG recordings, demographics, relevant clinical details of the acute presentation, and post-CM neurodevelopmental outcomes were determined during clinical follow-ups over a two-year period after discharge from the acute illness[11]. Neurodevelopmental outcomes captured included subsequent epilepsy, attention deficit disorder and hyperactivity (ADHD), and developmental disabilities. Seventy datasets were available and interpretable for computational EEG analyses for the present study through available software with mean ± SD recording time of 32 ± 4 minutes.
By the 2-year follow-up, 10 of the 70 CM survivors had developed epilepsy. All 70 EEGs were processed and included for frequency content analyses. A subset of EEGs were selected for conventional EEG analysis, consisting of the 10 subjects who developed epilepsy and 10 age- and sex-matched controls.
2.2. Ethical approval
The de-identified data for retrospective analysis were provided by GLB, principal investigator of the BMPES, under which the original data were collected and reported[11]. The original BMPES study was approved by the Research Ethics Committee at the University of Malawi College of Medicine and by the Biomedical Institutional Review Board at Michigan State University. Signed informed consent was obtained from parents or guardians of prospective participants prior to their enrollment in the study. The retrospective analyses were approved by the Institutional Review Board at Boston Children’s Hospital (protocol No.: IRB-P00029807).
2.3. EEG processing and analysis
EEG spectral analysis was performed using Persyst Software (version 13, Rev.E2, 2019.10.08). Raw time series EEG data were converted to frequency domain using FFT in 1 Hz frequency bins over 8-second time windows and power across the 1–60Hz range in delta (1–4 Hz), theta (5–7 Hz), alpha (8–12 Hz), beta (13–29 Hz), and gamma (30–60 Hz) frequency bands were initially analyzed. These initial analyses demonstrated that only power in the delta and gamma frequency bands differed significantly between the CM with epilepsy (CM+Epi) and CM without epilepsy (CM–Epi) groups; therefore, subsequent analyses were only focused on power within these bands. Mean spectral power in the delta and gamma frequency bands was normalized to the total power in the 1–60 Hz frequency range, to calculate relative spectral power.
A board-certified clinical neurophysiologist (AAP) reviewed the subset studies via Persyst software (version 13, Rev.E2, 2019.10.08) of CM survivors who developed epilepsy and of age- and sex-matched controls by conventional visual inspection, using a standardized form documenting presence or absence of seizures (focal or generalized), state change, slowing (focal or diffuse), and epileptiform activity (categorized as focal or generalized). The neurophysiologist was blinded to subject outcome (CM+Epi vs. CM-Epi) at the time of EEG review.
2.4. Statistical analyses
Comparisons of the categorical metrics between the CM+Epi and CM-Epi groups were performed by Fisher’s exact test. Continuous measures were compared between the two groups using independent-samples t-tests. Data analyses were performed using SPSS version 26 and Stata version 14.1.
Spectral EEG data were analyzed via Matlab 2016b for data manipulation and Stata 14.1 for statistical analyses. To identify predictors of post-CM epilepsy, we conducted logistic regressions with the dependent variable (DV) Epilepsy and potential independent variables (IVs) including Age (in months), Tmax (maximum Celsius temperature recorded during initial CM presentation), Coma Duration (in hours), Relative Gamma power, Relative Delta power, and Gamma-Delta (power) Ratio (unitless).
Potential IVs for inclusion in the multivariable logistic-regression analyses predicting the later development of post-CM epilepsy were selected following the recommendations of Bursac and colleagues[31]. We first conducted univariate logistic regressions for predicting epilepsy for each of the potential IVs listed above. IVs that had a univariate-regression p-value < 0.25 were selected for the multivariable logistic-regression modeling in the next step. To assess the predictive utility of the multivariable logistic-regression models, we calculated the area under the receiver operating characteristics (AUROC) curve [32] of select models. Further, to assess associations between the presence of ADHD and developmental disability/delay (Dev Delay) and post-CM epilepsy, we added ADHD and Dev Delay as IVs to the most-predictive multivariable model, i.e., the model with highest AUROC value. Finally, to assess robustness and overfitting of the multivariable regression models, we conducted k-fold cross-validation[33] (k = 4) for select models with the highest AUROC values.
3. Results
3.1. Demographics and initial clinical presentation
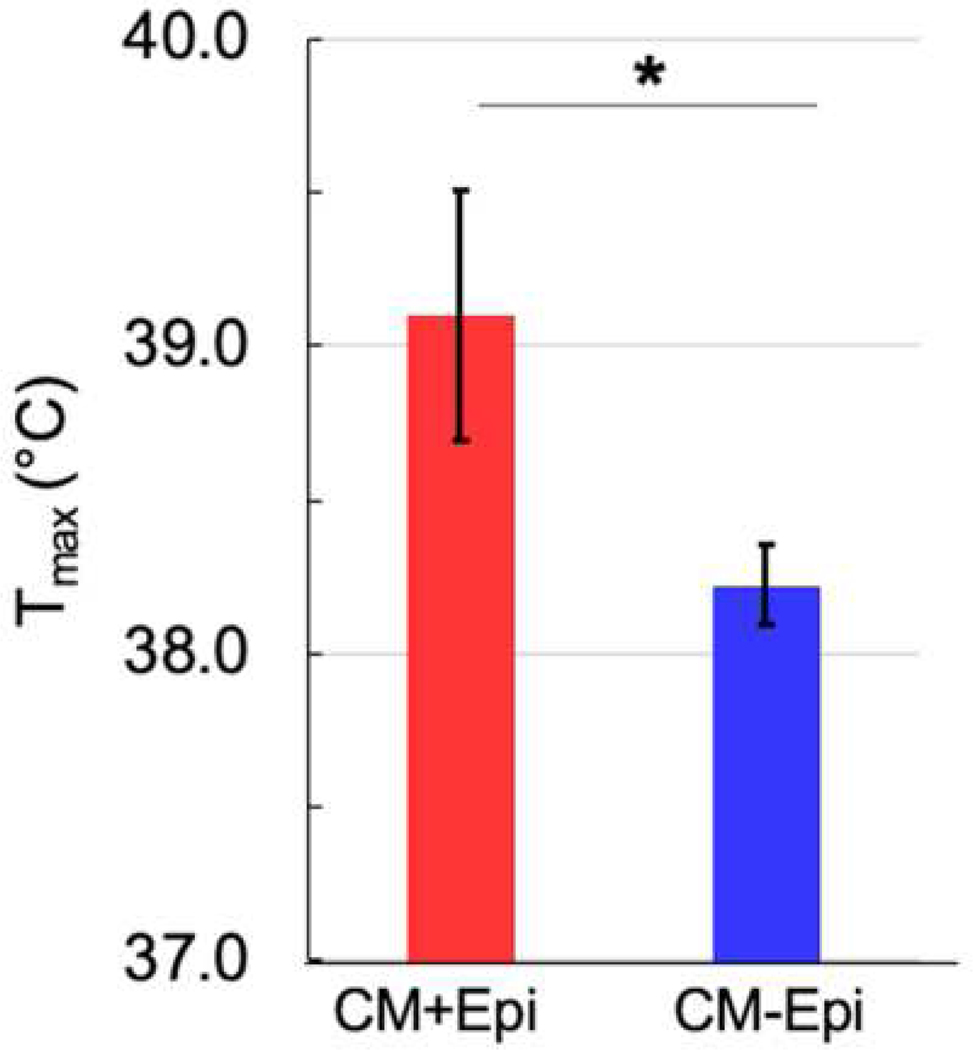
Demographic and initial clinical presentation data are detailed in Table 1. Age and sex were not statistically different between CM+Epi and CM–Epi groups. Coma duration and presence or absence of seizures during acute cerebral malarial infection were also not statistically different between the two groups. However, Tmax was significantly higher in the CM+Epi group than in the CM–Epi group (p=0.02), as reported previously[11] (Figure 1). Of note, comparison of Tmax between CM+Epi and CM–Epi groups found peak temperatures above ~38.5°C (upper limit of the 95% CI of Tmax in the CM–Epi group) substantially increases the risk of post-CM epilepsy.
Table 1.
Demographics and initial clinical presentation characteristics.
| Average Age (months) | Sex (M) | Tmax during CM(°C) | Coma duration during CM (days) | Number of patients with seizures on admission (%) | |
|---|---|---|---|---|---|
| CM-Epi (n=60) | 41.3 (SD=24.9) |
25 (42%) |
38.2 (SD=1.0) |
15.6 (SD =17.2) |
4 (7%) |
| CM+Epi (n=10) | 6.3 (SD=33.1) |
4 (40%) |
39.2* (SD=1.3) |
11.9 (SD=12.6) |
1 (10%) |
| Epilepsy alone (n=2) | 20.5 | 0 | 39 | 5 | 0 |
| Epilepsy + ADHD (n=3) | 38.3 | 2 | 39.7 | 7.5 | 0.3 |
| Epilepsy + Dev Delay (n=2) | 19 | 1 | 38.5 | 18 | 0 |
| Epilepsy + ADHD + Dev Delay (n=3) | 51 | 1 | 39 | 15 | 0 |
The only significant difference between two groups was in maximal temperature at presentation (p=0.02) (*)
Figure 1.
Tmax (mean ± SD) was significantly higher in the cerebral malaria survivors who developed epilepsy (CM+Epi) (39.1°C ± 1.3) than in those who did not (CM–Epi) (38.2°C ± 1.0), p = 0.02.
3.2. Epilepsy outcomes
Of the 70 subjects whose studies were available for analysis, 10 ultimately developed epilepsy. Within this group, 8 had an additional comorbid condition diagnosed within the follow-up period, 3 with ADHD, 2 with Dev Delay, and 3 with ADHD and Dev Delay.
3.3. Conventional EEG analysis
Visual EEG analysis performed on a subset of the standard routine EEG recordings obtained on initial presentation for CM revealed no significant differences between all 10 CM+Epi subjects and 10 age- and sex-matched CM-Epi controls who had no reported neurodevelopmental sequelae (Epilepsy, ADHD, or Dev Delay) within the 2-year follow-up. EEGs were assessed for presence or absence of the following parameters: absence of state change, focal slowing, diffuse slowing, focal spikes, generalized spikes, focal seizures, and generalized seizures. Overall, every EEG had at least one abnormality, and there was no significant difference in total number of abnormalities between the two groups. Interestingly, focal spikes were more commonly seen in the CM-Epi group compared to CM+Epi (40% vs 10%), with focal seizures seen equally in both groups (10%) and focal slowing and diffuse slowing with fairly similar distributions, as well. Absence of state change was seen in 40% of the CM+Epi group compared to 10% of the CM-Epi group. None of the between-group differences findings of conventional EEG analysis were statistically significant (Table 2).
Table 2.
Number of subjects with EEG abnormalities by visual inspection
| Focal seizures | Generalized seizures | Absence of State change | Focal slowing | Diffuse slowing | Focal spikes | Generalized spikes | |
|---|---|---|---|---|---|---|---|
| CM+Epi | 1 | 0 | 4 | 7 | 9 | 1 | 0 |
| CM-Epi | 1 | 0 | 2 | 5 | 10 | 5 | 0 |
Cerebral malaria survivors who developed epilepsy (CM+Epi) (n=10), did not differ significantly in any feature by visual EEG inspection in comparison to subset of matched controls (n=10), who did not develop epilepsy (CM-Epi).
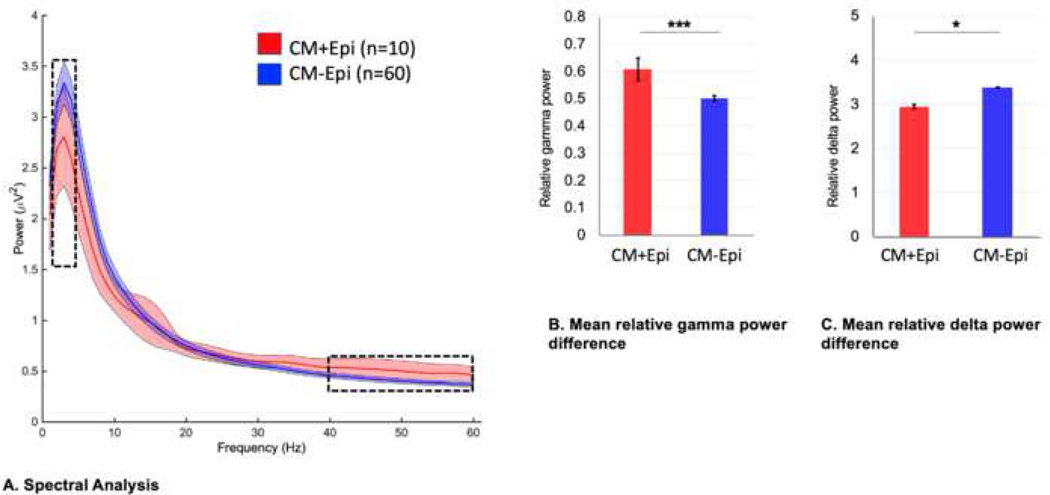
3.4. Spectral EEG analysis
Quantitative analysis was performed on the entire sample of 70 EEGs recorded during acute CM presentation (Figure 2). Aside from gamma and delta frequency bands, power within the other frequency bands did not differ significantly between the CM+Epi and CM–Epi groups (Figure 2A–B) and therefore analyses involving EEG spectral data were focused on delta and gamma power. Relative gamma power (mean ± SD) was significantly higher in the CM+Epi group (0.61 ± 0.13) than in the CM–Epi group (0.50 ± 0.08), p <0.001. Interestingly, relative delta power was significantly lower in the CM+Epi group (2.95 ± 0.75) than in the CM–Epi group (3.38 ± 0.56), p = 0.03. Accordingly, the gamma-delta power ratio was significantly higher in the CM+Epi group (0.23 ± 0.10) than in the CM–Epi group (0.16 ± 0.06), p = 0.003.
Figure 2.
(A) Spectral EEG analysis identifies a significant difference in gamma (30–60 Hz) and delta (1–4 Hz) frequency power bands during the acute CM phase between CM+Epi and CM–Epi groups. (B) Independent-samples t-tests found that the CM+Epi group had significantly higher relative gamma power (0.61 ± 0.13) compared to CM–Epi (0.50 ± 0.08), p<0.001, and (C) significantly lower relative delta power (2.95 ± 0.75) than the CM–Epi group (3.38 ± 0.56), p = 0.038. Error bars represent standard error of the mean, * p<0.05, ***p<0.001.
3.5. Predictive ability
Univariate logistic regressions for predicting post-CM epilepsy found significant p-values for Relative Gamma (β = 11.02, p = 0.003), Relative Delta (β = −1.13, p = 0.04), and Tmax (β = 0.77, p = 0.03), but not for Age (β = −0.01, p = 0.46) or Coma Duration (β = −0.02, p = 0.51). Thus, Relative Gamma, Relative Delta, and Tmax were entered as IVs into a multivariable logistic-regression model to predict post-CM epilepsy.
Significant effects were found for Relative Gamma (β = 14.37, p = 0.02) and Tmax (β = 0.84, p = 0.03), but not for Relative Delta (β = 0.50, p = 0.60), with AUROC = 0.830. Due to the large p-value for Relative Delta in the model, we repeated the multivariable analysis after excluding Relative Delta. The resultant model found significant effects for both Relative Gamma (β = 11.94, p = 0.003) and Tmax (β = 0.84, p = 0.03), with AUROC = 0.853, indicating that Relative Gamma was a better predictor of post-CM epilepsy than Relative Delta. A 4-fold cross-validation of this model found AUROC values ranging from 0.750 to 0.933, with a cross-validated AUROC of 0.825 (SD = 0.08). Of note, to evaluate for potential confounder effect of Tmax on Relative Gamma, the correlation coefficient between Tmax and Relative Gamma was calculated for both CM+Epi and CM-Epi groups. Neither coefficient was significant: CM+Epi r = −0.15, p = 0.68; CM-Epi r = −0.13, p = 0.32.
To assess whether gamma-delta power ratio was a better predictor of post-CM epilepsy than relative gamma power, we replaced Relative Gamma in the last model above with Gamma-Delta Ratio. The resultant model found significant effects for both Gamma-Delta Ratio (β = 11.37, p = 0.02) and Tmax (β = 0.84, p = 0.03), with AUROC = 0.820, indicating the higher predictiveness of the model including Relative Gamma compared to the models with either Gamma-Delta Ratio or Relative Delta.
When ADHD and Dev Delay were added as IVs to the best model (i.e., the model including Relative Gamma and Tmax), we found significant effects of Relative Gamma (β = 11.99, p = 0.01) and ADHD (β = 2.75, p = 0.01), a trend-level effect of Dev Delay (β = 1.80, p = 0.07), and a nonsignificant effect of Tmax (β = 0.58, p = 0.20), with AUROC = 0.920. A 4-fold cross-validation of this model found AUROC values ranging from 0.833 to 0.933, with a cross-validated AUROC of 0.877 (SD = 0.04). Receiver operating curve characteristics of the above variables are shown in Figure 3.
Figure 3.
Logistic-regression models predicting post-CM epilepsy, sorted in ascending order of predictiveness. The numbers indicate the area under the receiver operating characteristic (AUROC) curve associated with each model.
4. Discussion
This is the first study specifically utilizing spectral EEG analysis to assess predictive risk for epilepsy development in pediatric CM survivors. Our results indicate that during acute CM, children who will go on to develop post-CM epilepsy exhibit higher gamma-frequency EEG activity than those who do not. This fits with preclinical data indicating that the acute epileptogenic brain injury phase is characterized by excess extracellular glutamate, and likely hyperexcitation of inhibitory interneurons[34–36]. This hyperexcitation corresponds to an increase in gamma power on EEG, suggestive a final inhibitory-reserve expression before the ultimate degeneration of inhibitory interneurons[37, 38], likely from excitotoxic injury. Surprisingly, we also found that delta frequency band activity was significantly lower in the CM+Epi group, providing another potential metric of post-CM epileptogenesis and area for further investigation. However, in contrast to the mechanistic link between gamma oscillations and GABAergic inhibitory tone, an analogous link between delta oscillation and an epileptogenic pathology is not obvious. Nonetheless, we demonstrated the feasibility of extracting quantitative EEG data from studies acquired in sub-Saharan African countries where CM is endemic, which is an essential consideration for purposes of developing low-cost epileptogenesis biomarkers in resource-limited regions.
Our study expands upon the use of standard EEG recordings obtained from CM survivors and demonstrates the utility of spectral analyses in predicting post-CM epileptogenesis. Prior studies have described conventional EEG metrics in cerebral malaria as they relate to adverse outcomes, specifically mortality[18, 19, 39]. Diffuse background slowing, lower amplitude activity, poor reactivity and state change, asymmetry or focal slowing, and epileptiform features have all been reported in this population, which is a reflection of the acute illness. Significant differences between presence of increased delta slowing and lower amplitude has been reported as associated with death and early neurologic morbidity[18, 19]. However, there have been no studies looking at EEG metrics specifically in relationship to long-term neurodevelopmental sequelae, specifically post-CM epilepsy. Our study suggests that while conventional EEG findings from acutely ill children with CM demonstrate a mix of diffuse and focal slowing and epileptiform features, there is no significant difference in presence or absence of these findings between pediatric CM+Epi and CM–Epi survivors, thus confirming the current belief that conventional EEG analysis in acute brain insults is a poor predictor of future epilepsy development.
We have found, however, that the same EEG recordings can provide easily extractable data for quantitative analysis that does identify a metric for epilepsy risk prediction. Our study demonstrates a significantly higher level of gamma EEG activity in patients with acute CM who go on to develop epilepsy than in CM survivors who do not. However, as this gamma activity reflects the interneuron population expected to then subsequently deteriorate over time after cortical injury[20, 37], further prospective longitudinal studies looking at serial EEGs after recovery from acute CM through the period of epileptogenesis (i.e., until clinically apparent epilepsy) are needed to better understand this process.
Interestingly, we also found that delta activity in the acute phase was significantly lower in the pediatric CM+Epi group than in the CM–Epi group. This is unexpected given results from other, larger studies in which higher levels of low-amplitude delta activity on conventional EEG analysis were associated with higher risk of early neurologic morbidity[18]. Notably, those studies tend to include all CM patients while this study only assessed survivors. Additionally, those studies looked at early outcomes only, whereas our population was followed for two years. Thus, higher level of delta activity may reflect more profound acute injury from which recovery may be minimal. Among the children who later developed epilepsy in this study, 50% did not develop any developmental delay, suggesting that their disability was more moderate, and perhaps the initial injury was reflective of this. It is possible that delta activity reflects global cortical function in contrast to gamma activity, which appears to be more directly predictive of predisposition to epileptogenesis. Thus, serial EEG studies during the period of epileptogenesis would be useful in further understanding of this process.
Beyond the development of epilepsy, it has been shown in prior studies that CM is a significant risk factor for neurodevelopmental sequelae in general[11, 12]. Our data suggests that having another neurodevelopmental sequelae increases the risk of developing post-CM epilepsy. However, our sample size was too small to assess whether gamma-delta power ratio changes with the severity of neurodevelopmental outcomes or, more specifically, whether there is a significant difference in these quantitative EEG metrics between children who later develop epilepsy alone versus those who develop epilepsy along with other neurodevelopmental sequelae such as ADHD and/or developmental delay. Future studies investigating the relationship between the development of epilepsy and other comorbidities would be of interest, particularly in association with potential spectral EEG content predictors of post-CM epilepsy.
While the difference between gamma and delta power in pediatric CM survivors who later develop epilepsy and those who do not is promising as a potential biomarker of epilepsy outcomes, further studies assessing the changes in the power of frequency bands throughout the time period between acute insult and epilepsy development is required, as are validation studies, to determine the best utility EEG has in predicting post-CM epilepsy. In addition, this study suggests that additional clinical metrics may have predictive value that, when combined with quantitative EEG metrics (such as frequency content), could lead to a more comprehensive risk assessment. Thus, the most practical predictor of individual post-CM epilepsy risk is likely to be a combined model of specific clinical and EEG characteristics. Such a predictive risk model has great potential implications for clinical practice, allowing follow-up of the pediatric CM survivors most likely to develop epilepsy within a two-year period. Currently, follow-up of pediatric CM survivors is limited by resource constraints, and refinement of the population at highest risk could have significant implications for care.
The present study was limited by the fact that it was a retrospective analysis of EEGs obtained for a study performed on older software, leading to sample-size attrition due to processing issues and inability to obtain additional data outside the original study set. While we demonstrate the feasibility of this approach, a larger prospective trial to study the evolution of EEGs over time in this process as well as additional clinical factors that may have relevance in predicting post-CM risk are needed to further test these findings.
5. Conclusions
EEG metrics, either alone or in combination with clinical elements, can predict post-CM epilepsy and may provide practical, noninvasive, and affordable targets for biomarkers of epileptogenesis. Identifying predictive risk factors for post-CM epilepsy development has important implications. Alone, the condition leads to a significant burden of epilepsy in regions where resource allocation is essential, and stratification of risk could provide strategies for therapeutic or neuroprotective interventions. Additionally, cerebral malaria provides a unique model for studying acquired epilepsy and epileptogenesis, and identifying markers of epilepsy risk in this population has implications for future research in other acquired epilepsies.
Acknowledgements
The authors would like to thank the original BMPES study team for their generosity in making the data available to our team, and John Obrycki for assistance with data management for this study.
Funding
The EEG data analyzed in this publication were acquired during research supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (NIH) under Award Number K23NS046086 (GLB). AR and AJ were supported in part by the NIH R01 MH100186. AR was further supported by the NIH R01 NS088583, The Boston Children’s Hospital Translational Research Program, Autism Speaks, Massachusetts Life Sciences, The Assimon Family, Brainsway, CRE Medical, Eisai, Neuroelectrics, Roche, Sage Therapeutics, and Takeda Medical. MM was supported by NIH R01 ES026317. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard University and its affiliated academic health care centers, the National Institutes of Health, or any of the other listed granting agencies.
Abbreviations:
- CM
Cerebral Malaria
- FFT
fast Fourier transformation
- EEG
electroencephalogram
Footnotes
Conflict of Interests Statement
A.R. is a founder and advisor for Neuromotion, serves on the medical advisory board or has consulted for Cavion, Epihunter, Gamify, Neural Dynamics, NeuroRex, Roche, Otsuka, and is listed as inventor on a patent related to integration of TMS and EEG. The remaining authors have no conflicts of interest to disclose and declare that the research was conducted in the absence of any relevant commercial or financial relationships. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- [1].Collaborators GE. Global, regional, and national burden of epilepsy 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016 Lancet Neurol 2019;18: 357–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fiest K, Sauro KM, Wiebe S, Patten SB, Kwon C, Dykeman J, Pringsheim T, Lorenzetti DL, Jette N. Prevalence and Incidence of Epilepsy: A systematic review and meta-analysis of international studies Neurology 2017;88: 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Beghi E The Epidemiology of Epilepsy. Neuroepidemiology 2020;54: 185–191. [DOI] [PubMed] [Google Scholar]
- [4].Espinosa-Jovel C, Toledano R, Aledo-Serrano A, Garcia-Morales I, Gil-Nagel A. Epidemiological profile of epilepsy in low income populations. Seizure 2018;56: 67–72. [DOI] [PubMed] [Google Scholar]
- [5].Sueri C, Gasparini S, Balestrini S, Labate A, Gambardella A, Russo E, Leo A, Casarotto S, Pittau F, Trimboli M, Cianci V, Ascoli M, Cavalli SM, Ferrigno G, Aguglia U, Ferlazzo E. Diagnostic Biomarkers of Epilepsy. Curr Pharm Biotechnol 2018;19: 440–450. [DOI] [PubMed] [Google Scholar]
- [6].Pitkänen A, Löscher W, Vezzani A, Becker AJ, Simonato M, Lukasiuk K, Gröhn O, Bankstahl JP, Friedman A, Aronica E, Gorter JA, Ravizza T, Sisodiya SM, Kokaia M, Beck H. Advances in the development of biomarkers for epilepsy. The Lancet Neurology 2016;15: 843–856. [DOI] [PubMed] [Google Scholar]
- [7].Engel J Jr., Pitkanen A, Loeb JA, Dudek FE, Bertram EH 3rd, Cole AJ, Moshe SL, Wiebe S, Jensen FE, Mody I, Nehlig A, Vezzani A. Epilepsy biomarkers. Epilepsia 2013;54 Suppl 4: 61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Idro R, Jenkins NE, Newton CRJC. Pathogenesis, clinical features, and neurological outcome of cerebral malaria. The Lancet Neurology 2005;4: 827–840. [DOI] [PubMed] [Google Scholar]
- [9].Murphy SCB, JG. Gaps in the childhood malaria burden in Africa: Cerebral Malaria, Neurologic Sequelae, Anemia, Respiratory Distress, Hypoglycemia, and Complications of Pregnancy Am. J. Trop. Med. Hyg 2001;64: 57–67. [DOI] [PubMed] [Google Scholar]
- [10].WHO. WHO. Guidelines for the treatment of malaria, 2nd edition. 2010. In. Second ed. Geneva: WHO; 2010. [Google Scholar]
- [11].Birbeck GL, Molyneux ME, Kaplan PW, Seydel KB, Chimalizeni YF, Kawaza K, Taylor TE. Blantyre Malaria Project Epilepsy Study (BMPES) of neurological outcomes in retinopathy-positive paediatric cerebral malaria survivors: a prospective cohort study. The Lancet Neurology 2010;9: 1173–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Christensen SS, Eslick GD. Cerebral malaria as a risk factor for the development of epilepsy and other long-term neurological conditions: a meta-analysis. Trans R Soc Trop Med Hyg 2015;109: 233–8. [DOI] [PubMed] [Google Scholar]
- [13].Ngoungou EB, Koko J, Druet-Cabanac M, Assengone-Zeh-Nguema Y, Launay MN, Engohang E, Moubeka-Mounguengui M, Kouna-Ndouongo P, Loembe PM, Preux PM, Kombila M. Cerebral malaria and sequelar epilepsy: first matched case-control study in Gabon. Epilepsia 2006;47: 2147–53. [DOI] [PubMed] [Google Scholar]
- [14].Opoko RO, Bangirana P, Boivin M, John CC, Byarugaba J Seizure activity and neurological sequelae in Ugandan children who have survived an episode of cerebral malaria. African Health Sciences 2009;9: 75–81. [PMC free article] [PubMed] [Google Scholar]
- [15].Klein P, Dingledine R, Aronica E, Bernard C, Blumcke I, Boison D, Brodie MJ, Brooks-Kayal AR, Engel J Jr., Forcelli PA, Hirsch LJ, Kaminski RM, Klitgaard H, Kobow K, Lowenstein DH, Pearl PL, Pitkanen A, Puhakka N, Rogawski MA, Schmidt D, Sillanpaa M, Sloviter RS, Steinhauser C, Vezzani A, Walker MC, Loscher W. Commonalities in epileptogenic processes from different acute brain insults: Do they translate? Epilepsia 2018;59: 37–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Seydel KB, Kampondeni SD, Valim C, Potchen MJ, Milner DA, Muwalo FW, Birbeck GL, Bradley WG, Fox LL, Glover SJ, Hammond CA, Heyderman RS, Chilingulo CA, Molyneux ME, Taylor TE. Brain swelling and death in children with cerebral malaria. N Engl J Med 2015;372: 1126–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].IBE WI. Epilepsy: A public health imperative. 2019.
- [18].Postels DG, Wu X, Li C, Kaplan PW, Seydel KB, Taylor TE, Kousa YA, Idro R, Opoka R, John CC, Birbeck GL. Admission EEG findings in diverse paediatric cerebral malaria populations predict outcomes. Malar J 2018;17: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Crawley J, Smith S, Muthinji P, Marsh K, Kirkham F. Electroencephalographic and clinical features of cerebral malaria. Arch Dis Child 2001;84: 247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 2009;459: 698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nakamura T, Matsumoto J, Takamura Y, Ishii Y, Sasahara M, Ono T, Nishijo H. Relationships among parvalbumin-immunoreactive neuron density, phase-locked gamma oscillations, and autistic/schizophrenic symptoms in PDGFR-beta knock-out and control mice. PLoS One 2015;10: e0119258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 2009;459: 663–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hameed MQ, Hsieh TH, Morales-Quezada L, Lee HHC, Damar U, MacMullin PC, Hensch TK, Rotenberg A. Ceftriaxone Treatment Preserves Cortical Inhibitory Interneuron Function via Transient Salvage of GLT-1 in a Rat Traumatic Brain Injury Model. Cereb Cortex 2019;29: 4506–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hsieh TH, Lee HHC, Hameed MQ, Pascual-Leone A, Hensch TK, Rotenberg A. Trajectory of Parvalbumin Cell Impairment and Loss of Cortical Inhibition in Traumatic Brain Injury. Cereb Cortex 2017;27: 5509–5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lee S, Zhao X, Davis KA, Topjian AA, Litt B, Abend NS. Quantitative EEG predicts outcomes in children after cardiac arrest. Neurology 2019;92: e2329–e2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wiley SL, Razavi B, Krishnamohan P, Mlynash M, Eyngorn I, Meador KJ, Hirsch KG. Quantitative EEG Metrics Differ Between Outcome Groups and Change Over the First 72 h in Comatose Cardiac Arrest Patients. Neurocrit Care 2018;28: 51–59. [DOI] [PubMed] [Google Scholar]
- [27].Claassen J, Hirsch LJ, Kreiter KT, Du EY, Connolly ES, Emerson RG, Mayer SA. Quantitative continuous EEG for detecting delayed cerebral ischemia in patients with poor-grade subarachnoid hemorrhage. Clin Neurophysiol 2004;115: 2699–710. [DOI] [PubMed] [Google Scholar]
- [28].Taylor TE. Caring for children with cerebral malaria: insights gleaned from 20 years on a research ward in Malawi. Trans R Soc Trop Med Hyg 2009;103 Suppl 1: S6–10. [DOI] [PubMed] [Google Scholar]
- [29].Newton CRJC, Chokwe T, Schellenberg JA, Winstanley PA, Forster D, Peshu N, Kirkham FJ, Marsh K Coma scales for children severe falciparum malaria. Trans R Soc Trop Med Hyg 1997;91: 161–165. [DOI] [PubMed] [Google Scholar]
- [30].Society AE. Guideline one: minimum technical requirements for performing clinical electroencephalography. J Clin Neurophysiol 1994;11: 2–5. [PubMed] [Google Scholar]
- [31].Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med 2008;3: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Streiner DLCJ What’s under the ROC? An introduction to receiver operating characteristics curves. The Canadian Journal of Psychiatry. 2007;52: 121–8. [DOI] [PubMed] [Google Scholar]
- [33].Rodriguez JD, Perez A, Lozano JA. Sensitivity analysis of kappa-fold cross validation in prediction error estimation. IEEE Trans Pattern Anal Mach Intell 2010;32: 569–75. [DOI] [PubMed] [Google Scholar]
- [34].MacMullin P, Hodgson N, Damar U, Lee HHC, Hameed MQ, Dhamne SC, Hyde D, Conley GM, Morriss N, Qiu J, Mannix R, Hensch TK, Rotenberg A. Increase in Seizure Susceptibility After Repetitive Concussion Results from Oxidative Stress, Parvalbumin-Positive Interneuron Dysfunction and Biphasic Increases in Glutamate/GABA Ratio. Cerebral Cortex 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Parrish RR, Codadu NK, Mackenzie-Gray Scott C, Trevelyan AJ. Feedforward inhibition ahead of ictal wavefronts is provided by both parvalbumin- and somatostatin-expressing interneurons. The Journal of physiology 2019;597: 2297–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull 2008;34: 944–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Macmullin PC, Lee HH, Hodgeson N, Qiu J, Conley G, Morriss N, Mannix R and Rotenberg A Parvalbumin cell loss and extracellular glutamate/GABA imbalance in a mouse model of closed head injury. Neurotrauma 2018;35: A148–A148. [Google Scholar]
- [38].MacMullin P, Hodgson N, Damar U, Lee HHC, Hameed MQ, Dhamne SC, Hyde D, Conley GM, Morriss N, Qiu J Mannix R,Hensch T, Rotenberg A Increase in Seizure Susceptibility After Repetitive Concussion Results from Oxidative Stress, Parvalbumin-Positive Interneuron Dysfunction and Biphasic Increases in Glutamate/GABA Ratio. Cerebral Cortex. 2020. [DOI] [PMC free article] [PubMed]
- [39].Thumasupapong S, Tin T, Sukontason K, Sawaddichi C, Karbwang J Electroencephalography in Cerebral Malaria. Southeast Asian J Trop Med Public Health 1995;26: 34–37. [PubMed] [Google Scholar]