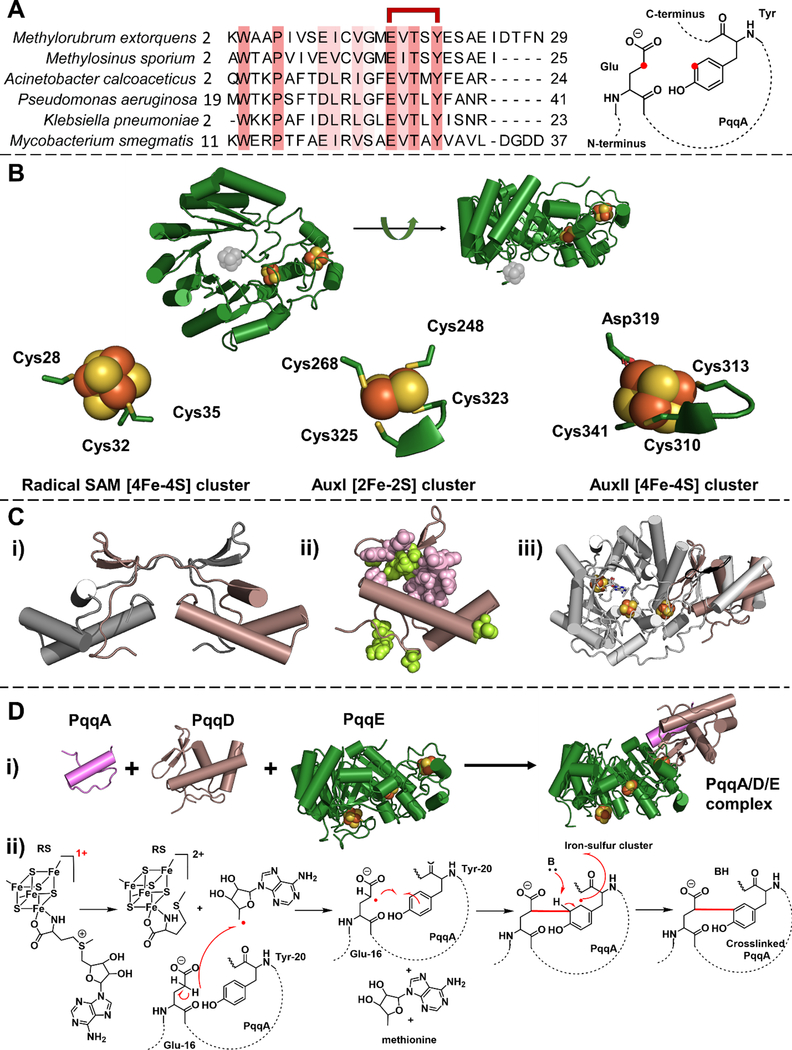
Figure 2. PqqA, PqqD and PqqE participate in the first step of the PQQ biogenesis.
A. PqqA is a short peptide. Left, the sequence alignment shows the conserved residues in PqqA (highlighted in salmon). The glutamate and tyrosine residues used to form PQQ are indicated as linked in red. Right, the representative structure of peptide PqqA. The to-be-crosslinked carbons on the glutamate and tyrosine residue are highlighted in red. B. X-ray crystal structure of Methylorubrum extorquens PqqE (PDB: 6C8V). The RS cluster in the N-terminal TIM barrel region was not well-resolved, possibly due to oxidative damage during the long delay times imposed by the protein crystallization process. The missing radical SAM [4Fe-4S] cluster is inserted (in gray). The ligands for the iron-sulfur cluster binding sites in PqqE are presented at the bottom. The ligand environment of the radical SAM [4Fe-4S] cluster is computationally modeled using CteB structure as a template by SWISS-MODEL.[62] C. Structures of PqqD. i) X-ray crystal structure of Xanthomonas campestris PqqD (PDB: 3G2B). A monomer of the PqqD dimer is shown in brown. ii) NMR structure of Methylorubrum extorquens PqqD (PDB: 3SXY) is shown in brown. The residues that interact with PqqA are highlighted in pink and the residues that interact with PqqE are highlighted in light green. iii) The PqqD NMR structure (brown) is superimposed with the peptide-binding domain in another radical SAM enzyme, CteB (gray) (PDB: 5WGG). D. The first step of PQQ biogenesis is catalyzed by a PqqA/D/E complex. i) A proposed structure for PqqA/PqqD/PqqE complex. The 3D structure of PqqA was predicted by PEP-FOLD3,[63] and the relative position of three components are modeled using CteB structure as a template by SWISS-MODEL.[62] ii) A proposed chemical mechanism of the reaction catalyzed in the PqqA/D/E complex. In the presence of PqqD and external reducing power, SAM is cleaved by PqqE, leading to the presumptive generation of 5’-deoxyadenosyl radical that abstracts a hydrogen atom from the γ-carbon of the conserved glutamate side chain of PqqA, to initiate C-C crosslinking to a conserved tyrosine.