Abstract
Objectives:
Psychological comorbidities have been associated with asthma in adults and children, but have not been studied in a population of children with severe asthma. The aim of this study was to test the hypothesis that symptoms of anxiety or depression are highly prevalent in pediatric severe asthma and negatively effects asthma control.
Methods:
Longitudinal assessments of anxiety or depression symptoms (Patient Health Questionnaire-4 (PHQ-4)), asthma control (Asthma Control Test (ACT)) and lung function were performed in a single-center pediatric severe asthma clinic. Participant data were collected during routine clinical care. Primary outcomes were ACT and forced expiratory volume in 1 second per forced vital capacity (FEV1/FVC).
Results:
Among 43 subjects (with total 93 observations), 58.1% reported at least 1 anxious or depressive symptom and 18.6% had a PHQ-4>2, the threshold for an abnormal test result. After adjusting for age, sex, race, and asthma medication step, there was a significant reduction in ACT for girls with PHQ-4>2 (adjusted mean (se) ACT for PHQ-4>2: 13.64 (0.59), ACT for PHQ-4≤2: 20.64 (1.25), p=0.02) but not boys. Moreover, there was a significant differential effect of mental health impairment for girls than boys. ACT for girls with PHQ>2: 13.64 (0.59) compared to boys with PHQ-4>2: 17.82 (0.95), adjusted mean difference ACT by sex = 4.18 points; 95% CI: 0.63, 7.73; p=0.033. In adjusted models, there was no association between PHQ-4>2 and FEV1/FVC.
Conclusions:
Symptoms of anxiety and depression are common. In children with severe asthma, a PHQ-4 score >2 is associated with worse asthma symptom control in girls, but not boys.
Keywords: Asthma, severe asthma, pediatrics, mental health, anxiety, depression, lung function, asthma control
Introduction
Asthma is the most common chronic respiratory condition in pediatric patients affecting approximately 9% of U.S children1. Severe asthma represents only 5–10% of the asthmatic population, but results in significantly higher morbidity and has a disproportionate burden on health care resources with asthma costs per patient being five times higher for patients with severe asthma compared to patients with mild to moderate asthma2,3. Comorbid conditions are common in children with severe asthma providing additional challenges to disease control. It is well known that mental health issues in chronic diseases have a strong bidirectional relationship with disease control4,5. The presence of psychiatric comorbidity in general pediatric asthma cohorts has been shown to increase symptom severity, reduce asthma control, lower quality of life and lower medication adherence,6 possibly mediated by heightened perception of respiratory symptoms7; however there are few data assessing anxiety and depression in children with severe asthma8,9. While evaluation of mental health comorbidities is highly recommended in the assessment of children with severe asthma10,11, there is little data specific to mental health comorbidities in the pediatric severe asthma population. Therefore, we aimed to determine the prevalence and character of mental health issues in pediatric severe asthma and its relationship to asthma control. We hypothesized that symptoms of anxiety and depression would be highly prevalent in our multidisciplinary pediatric severe asthma program and associated with poor asthma control and lung function.
Materials and Methods
Design
We performed a prospective quality improvement project to evaluate the prevalence of anxiety and depressive symptoms among patients attending the Boston Children’s Hospital multidisciplinary severe asthma clinic and their association with asthma control and lung function. All patients attending the Severe Asthma Program at Boston Children’s Hospital from March 2017 to December 2019 were encouraged to complete a four-question Patient Health Questionnaire (PHQ-4, Figure S-1)12 and the ACT13 or cACT14 as part of their clinical care. These were performed as part of the routine clinical patient assessment at each clinical encounter, both initial consultation and each follow-up visit.
Population
The Boston Children’s Hospital severe asthma program is a multidisciplinary program with a pediatric pulmonologist, pediatric allergist/immunologist, pediatric pulmonary nurse/asthma educator, and licensed social worker involved in the care of each patient with subspecialty consultants from otorhinolaryngology, gastroenterology and endocrinology, as needed. Patients are accepted to the clinic by referral from either a pediatric asthma specialist (allergist or pulmonologist) or pediatric intensivist after life-threatening asthma exacerbation, ensuring that the patient population is sufficiently difficult to manage or at-risk for severe asthma morbidity. The severe asthma program maintains an investigational review board (IRB)-approved clinical registry for patient characteristics, interventions and outcomes, for which all subjects in this study provided informed consent and assent for children 6 years of age and older. This study was approved by the Boston Children’s Hospital IRB. For the purpose of this analysis patients were included if they were age 4 years and older to ensure ability to measure asthma control by the validated childhood asthma control test (cACT)14,15.
Procedure
The PHQ-4 was used to assess symptoms of anxiety and depression. The PHQ-4 is a 4-item assessment that asks patients to rank, over the last two weeks, how often they have experienced specific symptoms of anxiety or depression with responses collected on a 4-point scale (see Figure S-1). The first two items are drawn from the ‘Generalized Anxiety Disorder–7 scale’ (GAD–7)16 and the second two items are drawn from the ‘Patient Health Questionnaire-8’ (PHQ-8)12, both self-administered validated population-based tools to identify anxiety and depression, respectively. A total score greater than 2 indicates at least mild impairment. The purpose of the PHQ-4 is to briefly and accurately assess patients for symptoms of depression and anxiety and prompt further assessment, if indicated. The PHQ-4 is validated down to 8 years of age; a small number of participants in our cohort were younger than this cutoff.
The primary outcome measures were the ACT or c-ACT, depending on age, and the ratio of forced expiratory volume in 1 second divided by the forced vital capacity (FEV1/FVC), the most sensitive marker of airflow obstruction in childhood asthma17. The ACT and the cACT were self-administered at the beginning of the clinical encounter. As with the PHQ-4, patients were instructed to self-report but may have had assistance from a parent. The ACT is comprised of five questions that ask patients to report on a 5-point scale, from the previous four weeks, how their asthma has hindered their activity level; induced shortness of breath; provoked nighttime awakenings; required the use of rescue medication; and from their own perception, has been controlled. The ACT can generate a potential score between 5 and 2513. The cACT assesses similar domains across 7 questions and generates scores ranging 0–2714. For both assessments, a score of greater than 19 indicates “well-controlled” asthma. Spirometry was performed according to standard guidelines18 on a rolling seal spirometer (Morgan Scientific, Hanover, MA, USA) and reviewed for quality. The global lung initiative19 predicted models were used to calculate percent predicted values for FEV1, FVC, and forced expiratory flow between the 25th-75th percentile (FEF25–75). FEV1/FVC was calculated from the best effort of each value and multiplied by 100 to generate a percentage for reporting. Demographic data was collected from the medical record for use in the asthma registry. Asthma medication step was determined from the patients active medication list at the time of each encounter and coded into step category to conform with current NHLBI categorizations20. In cases where the medications seemed to exceed one step but not achieve the next, for example a patient on medium dose inhaled corticosteroid/LABA combination, but also two other controller medications such as LAMA and LTRA, were considered in between step 3 and 4 and were given a numerical value of step 3.5 for use in analytic models.
Statistical Methods
Patient characteristics at the initial clinical encounter, and exposure/outcome measures across all observations, were summarized with descriptive statistics (count and percentage for categorical variables, mean with standard deviation for normally distributed continuous variables, and median with IQR for skewed continuous variables). Differences in PHQ-4 score by sex and age (dichotomized at 11 years to match the age cutoff between the cACT and ACT, the primary outcome) were assessed with Wilcoxon rank sum tests. All modeled statistical analyses included all available observations. To assess the association of PHQ-4 score > 2 with ACT score and PFT measures, we fit a generalized linear mixed model with a random effect for subject, compound symmetry covariance structure, and robust standard errors to correct for dependence among repeated observations. The interactions of PHQ-4 with age and sex were planned a priori and retained in the model if p<0.2 to maximize power21. All models were adjusted for sex, age, race (dichotomized as white vs. non-white due to small numbers), and NHLBI asthma medication step based on a priori assumptions from the asthma literature. FEV1/FVC ratios were scaled by 100%. A sensitivity analysis was performed in the sample limited to those observations in subjects 8 years old and older, the validated age cutoff for the PHQ-4 assessment tool. Results were considered significantly associated with outcome if p-values <0.05. SAS 9.4 (SAS Institute, Cary NC) was used in this analysis.
Results
Over the study period, complete data was collected for a total of 93 clinical encounters (observations) from 43 individual patients. All participants contributed at least one observation, 9 completed two observations; 5 completed three observations; 6 completed four observations; 2 completed five observations; and 1 completed six observations. Table 1 depicts subject characteristics of the cohort. The average participant age was 11.7 (± 4.2) years old and two thirds of the participants were male. The median NHLBI medication step was 5.5 (interquartile range (IQR) 4.5, 6.0), consistent with the diagnosis of severe asthma{Chung, 2014 #146} in the cohort. Overall, 58% of the participants had at least one symptom of anxiety and depression and 8 (18.6%) had PHQ-4>2. Across all observations, the median PHQ-4 score was 1 (IQR 0, 2), with 52.7% of observations reporting at least one symptom of anxiety or depression and 23% of observations meeting criteria for at least mild impairment with a PHQ score >2.
Table 1.
Subject characteristics
| Characterization | N = 43 subjects | |
|---|---|---|
| Age in Years, Mean (SD) | 11.7 (4.2) | |
| Sex, N (%) | Male | 28 (65.1%) |
| Female | 15 (34.9%) | |
| Race, N (%) | White | 21 (48.8%) |
| Black | 6 (14.0%) | |
| Asian | 1 (2.3%) | |
| Otder | 10 (23.3%) | |
| Unknown | 5 (11.6%) | |
| Medication Step, Median(IQR) | 5.5 (4.5, 6) | |
| PHQ-4 score ≥ 1 | 25 (58.1%) | |
| PHQ-4 score >2 | 8 (18.6%) | |
| Time-Varying Covariates | N = 93 observations | |
| ACT Score, Mean (SD) | 18.6 (4.9) | |
| ACT Score (4–11 Years), Mean (SD) | 18.9 (4.5) | |
| ACT Score (12+ Years), Mean (SD) | 18.1 (5.4) | |
| FEV1% Predicted, Mean (SD) | 95.1 (18.4) | |
| FVC% Predicted, Mean (SD) | 102.0 (14.3) | |
| FEF25–75% Predicted, Mean (SD) | 86.4 (35.7) | |
| FEV1/FVC, Mean (SD) | 81.0 (9.4) | |
| PHQ-4 Score, Median (IQR) | 1.0 (0.0, 2.0) | |
| PHQ-4 Score > 0, N(%) | 49 (52.7%) | |
| PHQ-4 Score > 2, N(%) | 21 (22.6%) |
Bivariate analysis of the continuous PHQ-4 and ACT scores demonstrated a moderate inverse correlation (Spearman correlation coefficient: r = −0.41, p<0.0001). After adjusting for age, sex, race, and medication step, we found significant sex differences between having a positive PHQ4 (PHQ-4 score >2) and ACT (Figure 1, Table 2). Mean ACT for girls with PHQ4 > 2 was 7 points lower relative to females with PHQ4 ≤ 2 (95% CI: −10.88, −3.12), which was significant (p=0.01), whereas, there was no significant difference in the mean ACT for boys with positive PHQ-4 scores than boys with PHQ-4≤2 (p = 0.84). Furthermore, there was a significant difference between boys and girls with positive scores, such that the mean ACT for boys with PHQ4 > 2 was 4.18 points higher relative to girls with PHQ4 > 2 (95% CI: 0.63, 7.73, p=0.03). There was no significant difference between ACT scores for boys and girls with PHQ4≤2 (p = 0.18). Importantly, there was no sex difference in the distribution of ACTs scores, suggesting that these findings were not due to overall differences in ACT between sexes.
Figure 1. Boxplot of ACT scores by sex and PHQ-4.
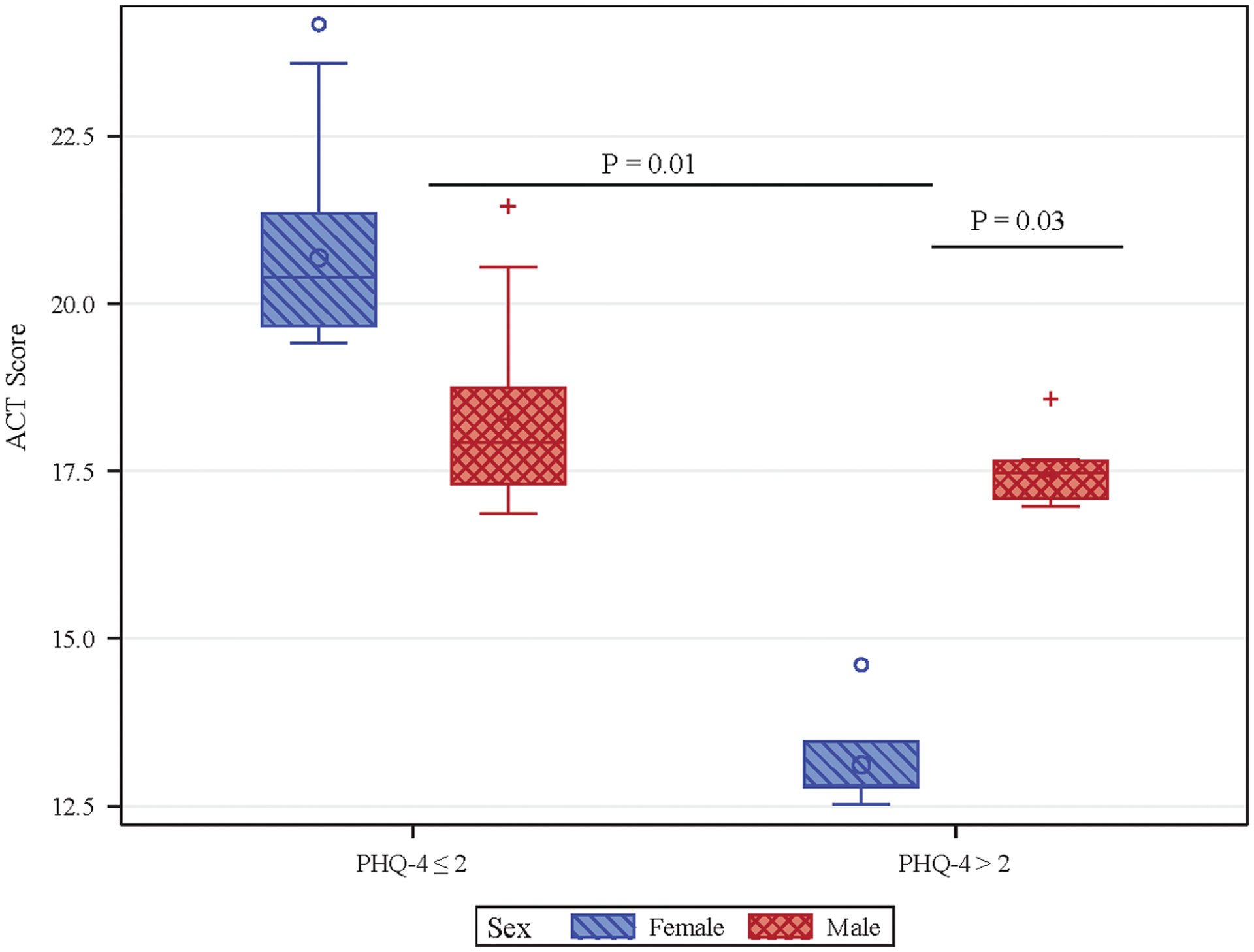
Boxplot of ACT scores stratified by sex and PHQ-4 >2 demonstrates significantly lower mean ACT for girls with PHQ-4>2 than boys with PHQ-4>2 (Adjusted mean & SE 13.64 (0.59) for females with PHQ4 > 2, 17.82 (0.95) for males with PHQ-4 > 2, mean difference 4.18 points (95% CI: 0.63, 7.73, p=0.03). Mean ACT is significantly lower girls with PHQ-4>2 than girls with PHQ-4 ≤ 2 (20.64 (1.25) for females with PHQ-4 ≤ 2, mean difference 7.0 points (95% CI: −10.88, −3.12, p=0.01). no significant difference in mean ACT scores for boys with PHQ-4 ≤ 2 (18.10 (0.75) and PHQ4 > 2 (mean difference 0.27 points (95% CI: −4.24, 3.69, p=0.8) or boys with PHQ-4 ≤ 2 vs. girls with PHQ-4 ≤ 2 (mean difference 2.55 points (95% CI: −7.19, 2.09, p=0.2). ACT, Asthma Control Test; PHQ-4, Patient Health Questionnaire-4.
Table 2.
Adjusted linear mixed model predicting ACT score from PHQ-4 > 2 (n=93)
| Model 1 | Model 2 | ||||||
|---|---|---|---|---|---|---|---|
| β | 95% CI | P-Value | β | 95% CI | P-Value | ||
| PHQ-4 > 2 | PHQ-4 > 2 | −2.18 | −6.04, 1.68 | 0.2 | −7.00 | −10.88, −3.12 | 0.01 |
| PHQ-4 ≤ 2 | 0 | 0 | |||||
| Sex | Male | −1.10 | −3.77, 1.58 | 0.4 | −2.55 | −5.50, 0.40 | 0.09 |
| Female | 0 | 0 | |||||
| Age | −0.08 | −0.43, 0.27 | 0.7 | −0.04 | −0.40, 0.31 | 0.8 | |
| Race | Non-White | 0.10 | −2.31, 2.50 | 0.9 | 0.11 | −2.32, 2.53 | 0.9 |
| White | 0 | 0 | |||||
| Medication Step | −0.94 | −2.02, 0.14 | 0.09 | −1.03 | −1.98, −0.08 | 0.04 | |
| PHQ-4 Score * Sex | Male, PHQ-4 > 2 | 6.73 | 1.73, 11.73 | 0.02 | |||
| Male, PHQ-4 ≤ 2 | 0 | ||||||
| Female, PHQ-4 > 2 | 0 | ||||||
| Female, PHQ-4 ≤ 2 | 0 | ||||||
Linear mixed effects model predicting ACT score by PHQ-4 >2. Model 1: main effects; model 2: addition of interaction effect of Sex on PHQ-4 >2 to predict ACT score.ACT: Asthma control test; PHQ-4: Patient Health Questionnaire-4
There was no association between having a positive PHQ-4 score and the primary spirometry outcome, FEV1/FVC, or FVC and FEF25–75% predicted (supplemental Table S-1). Surprisingly, there was a significant positive association with FEV1% predicted and PHQ-4>2 in adjusted models (supplemental Table S-1). None of the spirometry results demonstrated significant sex interactions.
A sensitivity analysis of the relationship between PHQ-4>2 and ACT restricted to children age 8 years and older confirmed the main analysis findings (supplemental Table S-2).
Discussion
Mental health impairment, particularly anxiety and depression, have been associated with poor asthma control22, but little is known about its prevalence and contribution to morbidity within the pediatric severe asthma population. Utilizing the brief PHQ-4 inventory for screening anxiety and depression in our pediatric severe asthma clinical cohort we found more than half of the patients reported at least one symptom of anxiety or depression and almost a quarter of the sample met clinically relevant criteria for at least mild mental health impairment.
We report the novel finding that among children with pediatric severe asthma, there is a sex difference in the relationship between mental health impairment, determined by a positive PHQ-4 score (>2) with perceived asthma control. We demonstrate a clinically meaningful significant reduction in girls reported ACT scores who had concurrent PHQ-4>2 compared to girls with PHQ-4 scores ≤2 and compared to boys with either high or low scores. Among boys, PHQ-4 score was not associated with any differences in ACT score.
Previous studies have identified the relationship between asthma and anxiety or depression leading to recommendations in national and international guidelines to consider comorbid mental health impairment in difficult asthma22–25. On the whole, the findings have been consistent that children with asthma have a higher prevalence of anxiety and depression compared to non-asthmatic control groups24–27, and anxiety and depression may be more prevalent the greater the asthma severity rating. A recent study by Montalbano and colleagues9 demonstrated that quality of life impairment and internalizing behavioral problems, especially the subscale including symptoms of anxiety and depressive symptoms, are more common in children with severe asthma than non-severe asthma. Notably, there was no sex differences identified in that cohort. There are several important findings highlighted by our study that address knowledge gaps in the understanding of mental health effects in children with severe asthma. First, few studies have included substantial numbers of patients with severe asthma. Here we evaluate the relationship between a self-reported measure of asthma control and lung function with mental health exclusively in pediatric patients with severe asthma. Our study design uses a repeated-measures analysis in order to capture a more dynamic profile of the relationship than cross-sectional measures. We also use patient reported symptoms that may vary between evaluations rather than static diagnoses. We demonstrate the quantitative relationship between symptoms of anxiety and depression and gradations of reported asthma control. We extend the findings of Richardson, et al. who identified increased asthma symptom reporting corresponding to increased anxiety or depressive symptoms in a general population of adolescents28 and more recently, Shams, et al, in black adolescents29, to a population of children with severe asthma, a group known to consume substantial healthcare resources.
Second, while evaluating mental health in patients with asthma has been identified as an important assessment step, there is little guidance on how to do so30. The bulk of the research to identify anxiety, depression or other internalizing and externalizing behaviors utilize exhaustive inventories ideal for use as research tools, but difficult to utilize in routine medical visits. Within our cohort we were able to capture clinically relevant information on mental health in a 4-question survey, easily administered in busy clinical practice, highly associated with an important health outcome.
Third, we found a significant interaction between female sex and asthma control, such that the overall association between mental health impairment and worse asthma control was significantly greater than the boys. Katon, et al. described female gender as an independent risk factor for the association of mood disorders and asthma diagnosis in adolescents24. Here, we extend these findings to include younger children with severe asthma with scaled tools to identify degree of mood disorder impairment and asthma control. Further, we demonstrate an interaction of sex and mood-asthma relationship that highlights the gender differences across severities of anxiety or depression and asthma control. However, this sex-specific finding was not found in a large meta-analysis including 3546 adolescents with asthma and 24,884 controls31, suggesting this finding requires further validation. While a recent study highlighted the hormonal basis for potential differences in lung function and asthma control in adolescents with severe asthma32, the mechanism by which these gender specific biochemical traits may influence the mental health-asthma morbidity relationship is still unclear. In our analysis, we specifically evaluated whether the association may have been a result of girls having lower ACT scores than boys, in general, but found no evidence to support a gender difference in the outcome measure.
One of the most surprising findings from this analysis was the marked discrepancy between the association of anxiety and depressive symptoms on asthma control measured by the asthma control test, a subjective measure of asthma impairment, with concordant lung function measures – an objective measure of asthma impairment – obtained at the same clinic visit. We found no relationship with airflow obstruction (FEV1/FVC), and an unexpected positive association with high PHQ-4 scores and FEV1 % predicted. Few adult studies, and no pediatric studies to our knowledge, have assessed lung function in the asthma – mood disorders relationship, with mixed findings33,34. There are several possibilities for the divergence of symptoms and lung function in our study. We believe this most likely highlights the influence of anxiety and depression on the perception of asthma control. Asthmatic patients with anxiety and depression are known to have higher rates of emergency department visits and care utilization35. Additionally, children and adults with anxiety are known to have altered perception of shortness of breath and higher degree of symptom reporting36. However, the bidirectional nature of the relationship is inherently present and difficult to disentangle, each condition exacerbating the other37. A recent qualitative analysis of the interaction between anxiety and asthma, Pateraki, Vance and Morris, describe three potential mechanisms contributing to the co-morbid conditions: asthma triggering unhelpful thinking and behavior that raises anxiety; anxiety impairing self-care and triggering hyperventilation; and both leading to self-perpetuating feedback cycles and symptom confusion38. Alternatively, phenotypic research in asthma highlights the multiple domains of asthma presentation and treatment responses with varying emphasis on lung function abnormalities, symptoms, inflammatory biomarkers and exacerbations39,40. Acknowledging these domains raises the possibility that those with symptom-dominant asthma are at higher risk of anxiety or depression. Further investigation to the manner in which mental health impairment may affect specific asthma outcome measures should be the focus of future research. However, it is important to recognize that it is the symptoms of asthma that are most burdensome to patients and drive resource utilization for asthma. Regardless of the nature of the asthma-mental health relationship, several studies suggest that addressing the mental health impairment can be effective in minimizing the mental health burden and improving asthma outcomes, with most evidence supporting directed cognitive behavioral therapy38,41,42. In high risk asthma populations, such as ours, patients’ mental health could improve if they are given access to mental health resources such as social workers, psychologists or therapists. Currently, we refer any child with a PHQ-4 >2 for formal mental health assessment.
We acknowledge that the PHQ-4 survey has limitations as an assessment tool and is not the gold standard for diagnosing anxiety or depression in clinical practice. Furthermore, some of our patients may have had formal diagnoses of anxiety or depression and scored low on the PHQ-4 due to successful treatment. With this in mind, the intention of this study was to evaluate the relationship of the symptoms of anxiety and depression with those of asthma control and lung function, which does not depend on the static designation of a mental health diagnosis. In fact, using this brief symptom-based assessment allows us to capture the variability of the symptoms and outcomes across several different time points. As with any study assessing sensitive information, mental health stigmatization may have introduced a reporting bias; patients or their guardians may have inaccurately reported the severity of their mental health impairment. Additionally, we used the PHQ-4 in some patients who were younger than the validation parameters. Whether it is as reflective of mental health conditions in children under 8 years old is unknown. The potential bias this might introduce is likely to be minimal as the questions are approachable to children of all ages and within our analyses we did not find age to function as either a major confounder or a modifier of the relationship between PHQ-4 and asthma outcomes. Further, our sensitivity analysis restricted to subjects 8 years and older demonstrated similar findings to the cohort as a whole. We acknowledge that this data was collected as part of clinical care and may lack some of the rigor of prospective epidemiologic clinical research, but does represent a real-world finding and clinically relevant information that is able to be collected as part of routine care. This research was specifically targeted to a high-risk cohort that was highly selected and relatively small in number which may limit its applicability to patients beyond those identified with severe asthma outside of this referral population.
There is a high rate of mental health impairment among pediatric patients with severe asthma. We found that increasing symptoms of anxiety or depression on a simple 4-question clinical survey was highly associated with report of poor symptomatic asthma control but not decreased lung function, among girls. Assessing pediatric patients with severe asthma for psychiatric problems is important in order to uncover a potentially modifiable comorbidity for poor asthma control. Further research to test the efficacy of mental health interventions to improve asthma morbidity will be important.
Supplementary Material
Figure S-1. Patient Health Questionnaire
Funding sources:
This study was supported by NIH grants K23AI106945, R01 ES 030100, Boston Children’s Hospital Department of Medicine Quality Program (PI Gaffin).
Footnotes
Conflict of interest: No authors have any conflicts of interest to report.
References
- 1.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics 2009;123 Suppl:S131–45. [DOI] [PubMed] [Google Scholar]
- 2.Nurmsagambetov T, Kuwahara R, Garbe P. The Economic Burden of Asthma in the United States, 2008–2013. Ann Am Thorac Soc 2018;15(3):348–356. [DOI] [PubMed] [Google Scholar]
- 3.Zeiger RS, Schatz M, Dalal AA, Qian L, Chen W, Ngor EW, Suruki RY, Kawatkar AA. Utilization and Costs of Severe Uncontrolled Asthma in a Managed-Care Setting. J Allergy Clin Immunol Pr 2016;4(1):120–9 e3. [DOI] [PubMed] [Google Scholar]
- 4.Chapman DP, Perry GS, Strine TW. The vital link between chronic disease and depressive disorders. Prev Chronic Dis 2005;2(1):A14. [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandes L, Fonseca J, Martins S, Delgado L, Pereira AC, Vaz M, Branco G. Association of anxiety with asthma: subjective and objective outcome measures. Psychosomatics 2010;51(1):39–46. [DOI] [PubMed] [Google Scholar]
- 6.Del Giacco SR, Cappai A, Gambula L, Cabras S, Perra S, Manconi PE, Carpiniello B, Pinna F. The asthma-anxiety connection. Respir Med 2016;120:44–53. [DOI] [PubMed] [Google Scholar]
- 7.Feldman JM, Steinberg D, Kutner H, Eisenberg N, Hottinger K, Sidora-Arcoleo K, Warman K, Serebrisky D. Perception of pulmonary function and asthma control: the differential role of child versus caregiver anxiety and depression. J Pediatr Psychol 2013;38(10):1091–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montella S, Baraldi E, Cazzato S, Aralla R, Berardi M, Brunetti LM, Cardinale F, Cutrera R, De Benedictis FM, Di Palmo E, et al. Severe asthma features in children: A case–control online survey. Ital J Pediatr 2016. [accessed 2020 Sep 9];42(1). https://pubmed.ncbi.nlm.nih.gov/26796331/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montalbano L, Ferrante G, Montella S, Cilluffo G, Di Marco A, Bozzetto S, Di Palmo E, Licari A, Leonardi L, Caldarelli V, et al. Relationship between quality of life and behavioural disorders in children with persistent asthma: a Multiple Indicators Multiple Causes (MIMIC) model. Sci Rep 2020. [accessed 2020 Sep 9];10(1). https://pubmed.ncbi.nlm.nih.gov/32332757/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barsky EE, Giancola LM, Baxi SN, Gaffin JM. A Practical Approach to Severe Asthma in Children. Ann Am Thorac Soc 2018;15(4):399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bush A, Saglani S. Management of severe asthma in children. Lancet (London, England) 2010;376(9743):814–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kroenke K, Spitzer RL, Williams JB, Lowe B. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics 2009;50(6):613–621. [DOI] [PubMed] [Google Scholar]
- 13.Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, Murray JJ, Pendergraft TB. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol 2004;113(1):59–65. [DOI] [PubMed] [Google Scholar]
- 14.Liu AH, Zeiger R, Sorkness C, Mahr T, Ostrom N, Burgess S, Rosenzweig JC, Manjunath R. Development and cross-sectional validation of the Childhood Asthma Control Test. J Allergy Clin Immunol 2007;119(4):817–825. [DOI] [PubMed] [Google Scholar]
- 15.Schatz M, Sorkness CA, Li JT, Marcus P, Murray JJ, Nathan RA, Kosinski M, Pendergraft TB, Jhingran P. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol 2006;117(3):549–556. [DOI] [PubMed] [Google Scholar]
- 16.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166(10):1092–1097. [DOI] [PubMed] [Google Scholar]
- 17.Strunk RC, Weiss ST, Yates KP, Tonascia J, Zeiger RS, Szefler SJ, Group CR. Mild to moderate asthma affects lung growth in children and adolescents. J Allergy Clin Immunol 2006;118(5):1040–1047. [DOI] [PubMed] [Google Scholar]
- 18.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. Standardisation of spirometry. Eur Respir J 2005;26(2):319–338. [DOI] [PubMed] [Google Scholar]
- 19.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MS, Zheng J, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012;40(6):1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.EPR3 N, Services USD of H and H. Guidelines for the Diagnosis and Management of Asthma. NIH; 2007. [Google Scholar]
- 21.Ulm K Statistical analysis of epidemiologic data Steve Selvin, Oxford University Press, Oxford, 1991. No Of Pages: xiv + 375. Price: £27.50. ISBN: 0-19-506766-5. Stat Med 1994. [accessed 2020 Sep 7];13(10):1083–1083. http://doi.wiley.com/10.1002/sim.4780131010 [Google Scholar]
- 22.Sastre J, Crespo A, Fernandez-Sanchez A, Rial M, Plaza V. Anxiety, Depression, and Asthma Control: Changes After Standardized Treatment. J Allergy Clin Immunol Pr 2018;6(6):1953–1959. [DOI] [PubMed] [Google Scholar]
- 23.Galić K, Dodaj A, Ćorluka-Čerkez V, Lasic V, Pejić R, Šimić J, Vukojević M. Study of depression and anxiety in patients with asthma and chronic obstructive pulmonary disease. Psychiatr Danub 2019;31(Suppl 1):112–117. [PubMed] [Google Scholar]
- 24.Katon W, Lozano P, Russo J, McCauley E, Richardson L, Bush T. The prevalence of DSM-IV anxiety and depressive disorders in youth with asthma compared with controls. J Adolesc Heal 2007;41(5):455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kewalramani A, Bollinger ME, Postolache TT. Asthma and Mood Disorders. Int J Child Heal Hum Dev 2008;1(2):115–123. [PMC free article] [PubMed] [Google Scholar]
- 26.Goodwin RD, Messineo K, Bregante A, Hoven CW, Kairam R. Prevalence of probable mental disorders among pediatric asthma patients in an inner-city clinic. J Asthma 2005;42(8):643–647. [DOI] [PubMed] [Google Scholar]
- 27.Morrison KM, Goli A, Van Wagoner J, Brown ES, Khan DA. Depressive Symptoms in Inner-City Children With Asthma. Prim Care Companion J Clin Psychiatry 2002;4(5):174–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson LP, Lozano P, Russo J, McCauley E, Bush T, Katon W. Asthma symptom burden: relationship to asthma severity and anxiety and depression symptoms. Pediatrics 2006;118(3):1042–1051. [DOI] [PubMed] [Google Scholar]
- 29.Shams MR, Bruce AC, Fitzpatrick AM. Anxiety Contributes to Poorer Asthma Outcomes in Inner-City Black Adolescents. J Allergy Clin Immunol Pr 2018;6(1):227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014;43(2):343–373. [DOI] [PubMed] [Google Scholar]
- 31.Lu Y, Mak KK, van Bever HP, Ng TP, Mak A, Ho RC. Prevalence of anxiety and depressive symptoms in adolescents with asthma: a meta-analysis and meta-regression. Pediatr Allergy Immunol 2012;23(8):707–715. [DOI] [PubMed] [Google Scholar]
- 32.DeBoer MD, Phillips BR, Mauger DT, Zein J, Erzurum SC, Fitzpatrick AM, Gaston BM, Myers R, Ross KR, Chmiel J, et al. Effects of endogenous sex hormones on lung function and symptom control in adolescents with asthma. BMC Pulm Med 2018;18(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodwin RD, Chuang S, Simuro N, Davies M, Pine DS. Association between lung function and mental health problems among adults in the United States: findings from the First National Health and Nutrition Examination Survey. Am J Epidemiol 2007;165(4):383–388. [DOI] [PubMed] [Google Scholar]
- 34.Rimington LD, Davies DH, Lowe D, Pearson MG. Relationship between anxiety, depression, and morbidity in adult asthma patients. Thorax 2001;56(4):266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bardach NS, Neel C, Kleinman LC, McCulloch CE, Thombley R, Zima BT, Grupp-Phelan J, Coker TR, Cabana MD. Depression, Anxiety, and Emergency Department Use for Asthma. Pediatrics 2019;144(4). [DOI] [PubMed] [Google Scholar]
- 36.Urrutia I, Aguirre U, Pascual S, Esteban C, Ballaz A, Arrizubieta I, Larrea I. Impact of anxiety and depression on disease control and quality of life in asthma patients. J Asthma 2012;49(2):201–208. [DOI] [PubMed] [Google Scholar]
- 37.Leander M, Lampa E, Rask-Andersen A, Franklin K, Gislason T, Oudin A, Svanes C, Toren K, Janson C. Impact of anxiety and depression on respiratory symptoms. Respir Med 2014;108(11):1594–1600. [DOI] [PubMed] [Google Scholar]
- 38.Pateraki E, Vance Y, Morris PG. The Interaction Between Asthma and Anxiety: An Interpretative Phenomenological Analysis of Young People’s Experiences. J Clin Psychol Med Settings 2018;25(1):20–31. [DOI] [PubMed] [Google Scholar]
- 39.Bossley CJ, Fleming L, Ullmann N, Gupta A, Adams A, Nagakumar P, Bush A, Saglani S. Assessment of corticosteroid response in pediatric patients with severe asthma by using a multidomain approach. J Allergy Clin Immunol 2016;138(2):413–420.e6. [DOI] [PubMed] [Google Scholar]
- 40.Fitzpatrick AM, Moore WC. Severe Asthma Phenotypes - How Should They Guide Evaluation and Treatment? J Allergy Clin Immunol Pr 2017;5(4):901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiang LC, Ma WF, Huang JL, Tseng LF, Hsueh KC. Effect of relaxation-breathing training on anxiety and asthma signs/symptoms of children with moderate-to-severe asthma: a randomized controlled trial. Int J Nurs Stud 2009;46(8):1061–1070. [DOI] [PubMed] [Google Scholar]
- 42.Marriage D, Henderson J. Cognitive behaviour therapy for anxiety in children with asthma. Nurs Child Young People 2012;24(9):30–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S-1. Patient Health Questionnaire


