Figure 1. The cardiac sarcomere and assembly of the myofilaments.
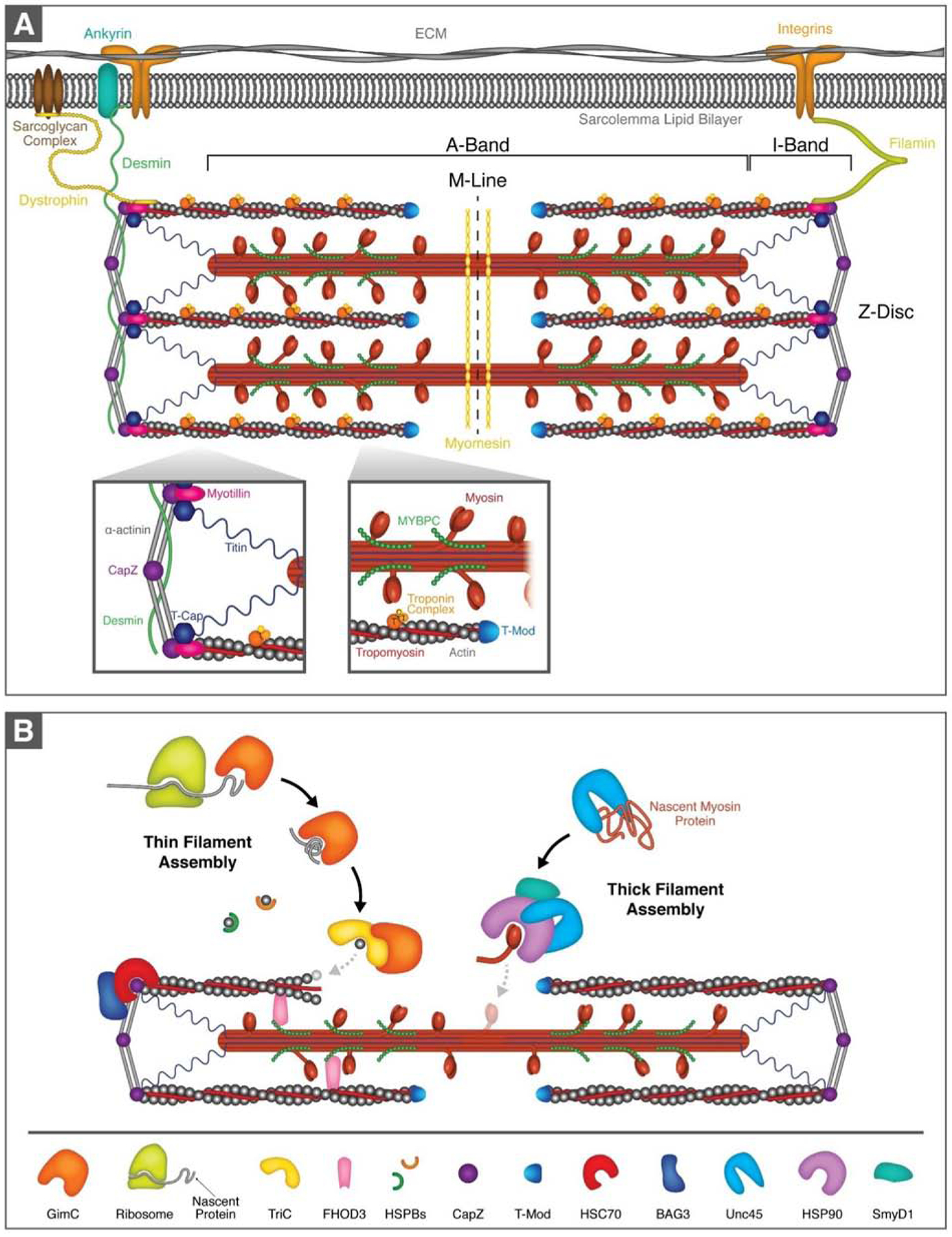
(A).Schematic diagram of the sarcomere and sarcomere-associated proteins. The sarcomere is the basic contractile unit in striated muscle with the thin actin and thick myosin myofilament proteins at its core. Sarcomeres are bounded at either end by α-actinin-rich Z-discs and are arranged sequentially to form myofibrils. (B). Simplified schematic of thin and thick filament assembly. GimC binds newly synthesized actin as it is translated by the ribosome and mediates early stages of folding. GimC passes now semi-folded actin to TriC, which assists in the assembly of the actin filament. Thin filament assembly is further supported by FHOD3, which stabilizes monomeric and filamentous actin and is crucial for actin filament nucleation and polymerization. The small heat shock proteins (heat shock protein B family, HSPBs) stabilize actin monomers and prevent protein aggregate formation. Actin capping proteins, CapZ and tropomodulin (T-Mod), bind to the ends of actin filaments and maintain stability. CapZ itself requires the BAG3/HSC70 chaperone complex to maintain its stability. Unc45 is the key chaperone for thick filament assembly and stability. It prevents myosin aggregation and assists with the folding of the myosin ATPase domain. Unc45 is a co-chaperone for HSP90, which participates in the early stages of myosin folding and myofibril assembly. The lysine methyltransferase SmyD1 is proposed to interact with the HSP90/Unc45/myosin complex.
