Figure 3. Chaperone-assisted selective autophagy at the sarcomere.
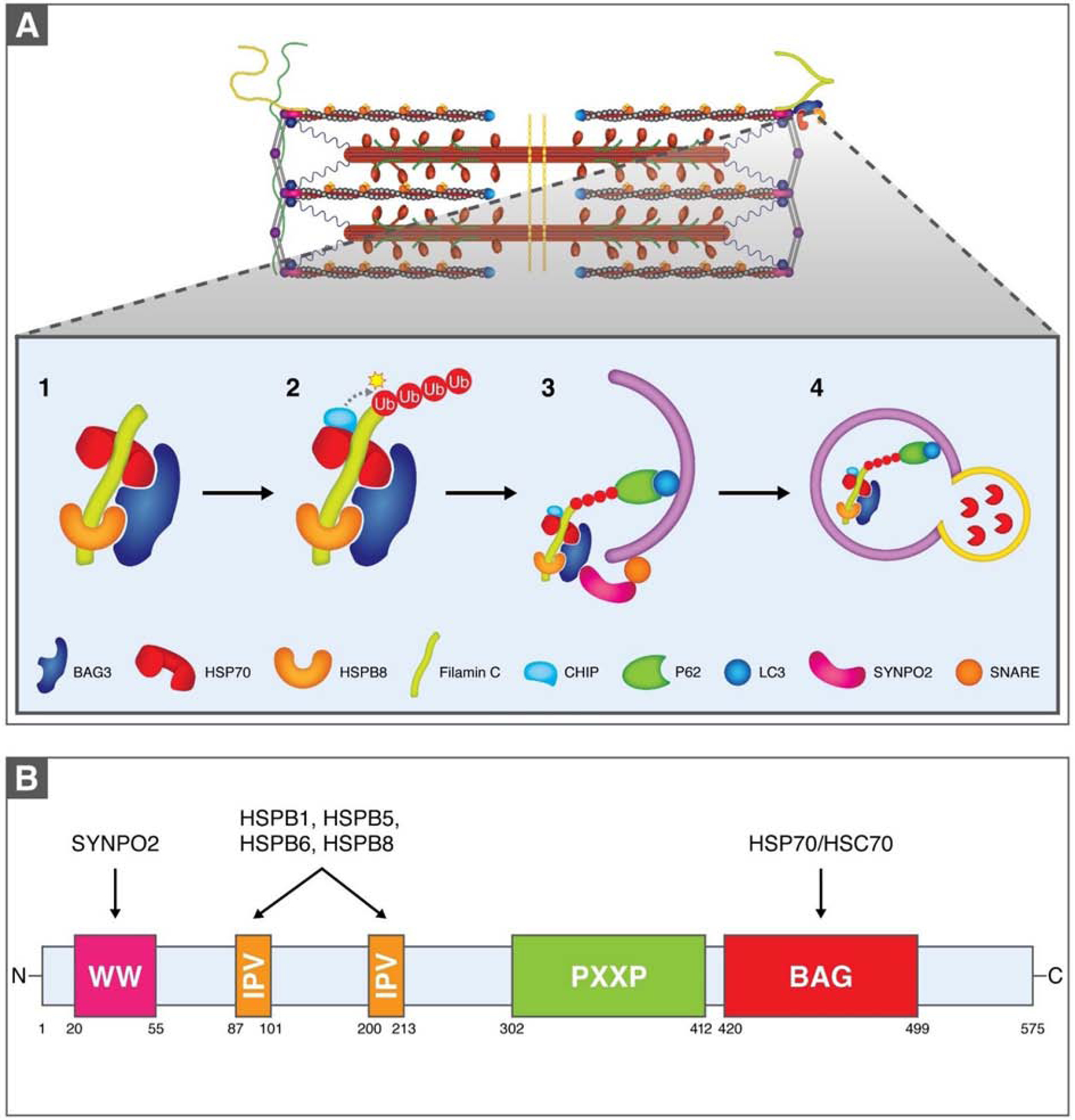
(A). The co-chaperone BAG3 operates as a scaffold for HSP70 and HSPB8, which bind to misfolded filamin C. The E3 ligase CHIP then ubiquitinates filamin C, allowing it to be recognized by the ubiquitin receptor P62, which facilitates the association of the CASA complex with LC3 on the autophagosome membrane. Through BAG3 this complex also associates with SYNPO-2, which mediates interaction with a SNARE protein on the autophagosome membrane that assists with autophagosome formation. Autophagosome contents are recycled via autophagosome-lysosome fusion. (B). BAG3 domains enable numerous binding activities. SYNPO2, which assists in autophagosome formation, binds to the N-terminal WW domain. Several HSPBs bind to two IPV motifs and the BAG domain associates with the ATPase domain of HSP70/HSC70.
