Figure 1.
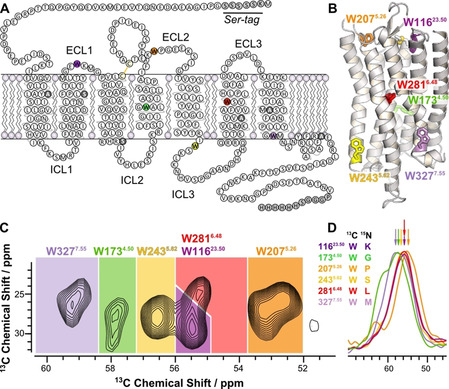
Sequence and structural architecture of Y2R and assignment of the 13Cα‐Trp signals of Y2R in DMPC membranes. A) Snake plot showing the Y2R construct used in this study with artificial amino acid insertions at the N‐ and C‐termini (gray). Cysteines not involved in disulfide bond formation were replaced by Ala or Ser (dark gray). The six 13C labeled Trp are colored. B) Structural model of Y2R [23] with the Trp residues represented as stick models. C) Cα/Cβ region of a 13C‐13C DARR NMR spectrum of Y2R recorded at a temperature of −30 °C with the assignment to the specific residues. D) Cα region of the DNP NCOCX spectra used for signal assignment. NMR spectra were recorded using DNP for signal enhancement at a temperature of −164 °C. The labeling scheme for the sample preparation indicating the 13C‐labeled Trp and its 15N‐labeled successor amino acid is given. See also Supplementary Figure S2.
