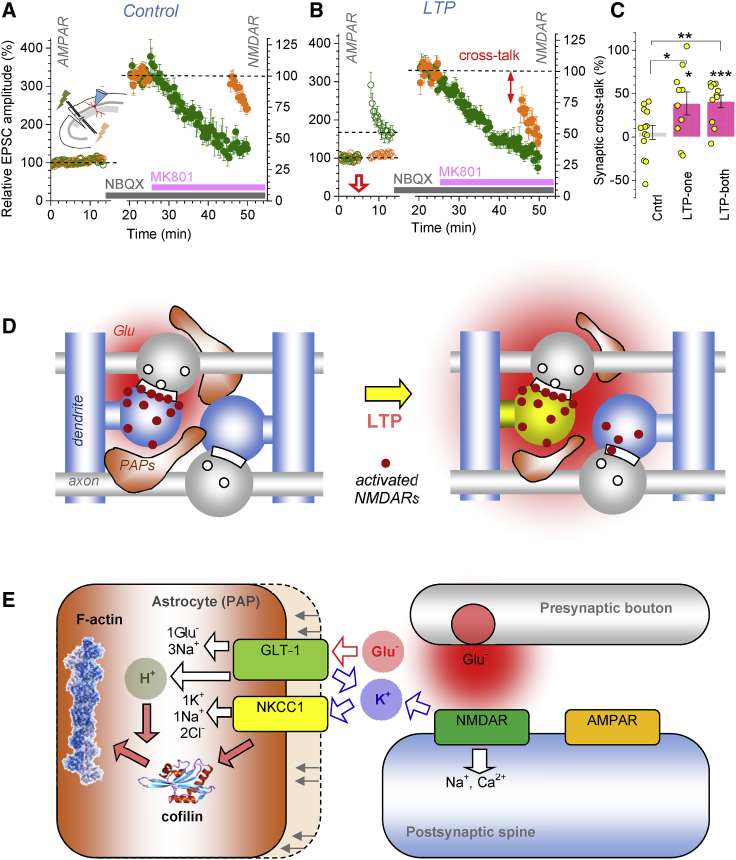
Figure 8.
LTP Induction Boosts NMDAR-Mediated Inter-synaptic Cross-Talk
(A) Inset diagram, experiment design to test NMDAR-mediated cross-talk between two afferent pathways (green and orange lightning) (Scimemi et al., 2004) (Figure S8A; STAR Methods). Plot, relative EPSC amplitude (mean ± SEM, n = 13), with single stimuli, 20 s apart, applied alternately to the two pathways (green and orange). AMPAR EPSCs are recorded for 12–15 min (Vm = −70 mV; left ordinate), then NMDAR EPSCs for ~5 min (10 μM NBQX, Vm = −20 mV; right ordinate). Once MK801 is added, NMDAR EPSCs are recorded in active (green) pathway only. Resuming stimulation in the silent (orange) pathway reveals little change in the NMDAR EPSC amplitude compared to baseline (dotted line).
(B) Experiment as in (A) but with LTP induced in the active pathway (red arrow; n = 7). Reduced NMDAR EPSCs in the silent (orange) pathway upon resumed stimulation (arrow, cross-talk) point to NMDAR activation by glutamate escaping from the active (green) pathway.
(C) Summary of experiments in (A) and (B). The degree of cross-talk (percentage of one-pathway NMDARs activated by glutamate discharges at the other pathway; mean ± SEM), in control (Cntrl, n = 13), with LTP induced either in one (LTP-one, n = 10) or both (LTP-both, n = 11; Figures S8C and S8D) pathways, prior to NMDAR EPSC recordings; ∗p < 0.05 (df = 21 for Cntrl versus LTP-one), ∗∗p < 0.01 (df = 22), ∗∗∗p < 0.005.
(D) Proposed changes in PAPs after LTP induction. In baseline conditions (left), PAPs restrict glutamate action to the synaptic cleft and some extrasynaptic NMDARs (red dots). After LTP induction (right), some PAPs withdraw, widening the pool of activated extrasynaptic NMDARs, including neighboring synapses.
(E) Diagram, candidate cellular mechanisms of LTP-driven PAP withdrawal. LTP induction activates postsynaptic NMDARs and engages GLT1 transporters. This generates an extracellular K+ hotspot, activating the NKCC1-cofilin-1 pathway that engages, in a pH-sensitive manner, actin polymerization responsible for morphogenesis.