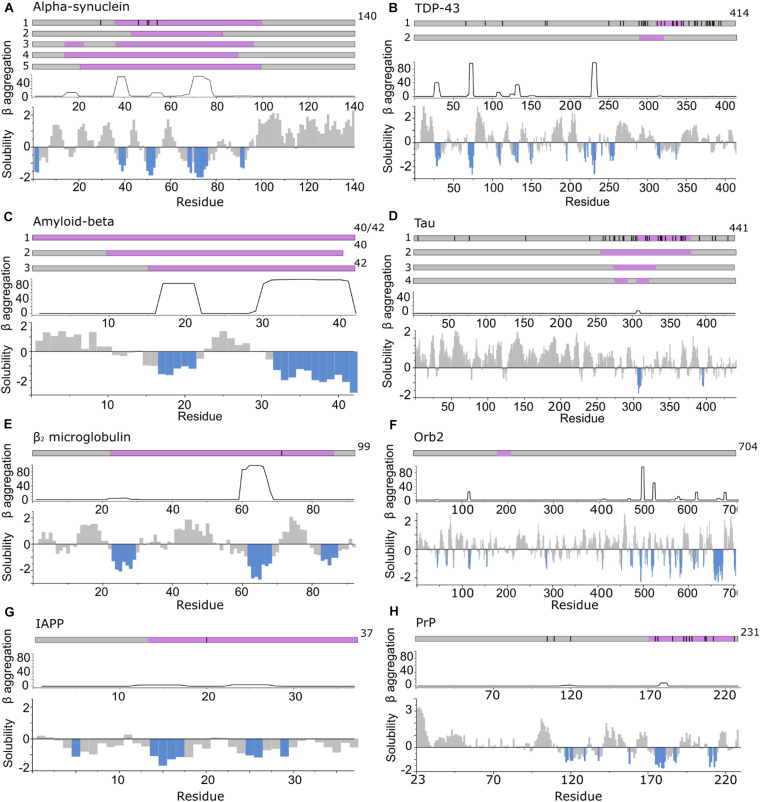
FIGURE 3.
APRs comprise only a small part of the amyloid core. Top in A–H: location of fibril cores of αSyn, TDP-43, Aβ, Tau, β2m, Orb2B, IAPP, and PrP defined by recent cryoEM or ssNMR fibril structures (purple). The positions of familial disease mutations are highlighted where appropriate as black lines. Bottom in A–H: regions with low solubility predicted by CamSol (below –1 is aggregation promoting highlighted in blue) (Sormanni et al., 2015) and the β-aggregation potential of each sequence predicted using TANGO (Fernandez-Escamilla et al., 2004). (A) αSyn including polymorph 1a (1) (Guerrero-Ferreira et al., 2018; Li B. et al., 2018; Li Y. et al., 2018) (core residues 37–99), 1b (2) (Li B. et al., 2018) (core residues 43–83), 2a and b (3) (Guerrero-Ferreira et al., 2019) (core residues 14–24, 36–96) and the MSA ex vivo structures including residues 14–94 (for PF-IA and PF-IIA) (4) or residues 21–99 (for PF-IB and PF-IIB) (5) in the fibril core (Schweighauser et al., 2020). (B) TDP-43 cryoEM structure solved from C-terminal segments forming a dagger shaped core (1) (residues 312–346) or R-shaped core (2) (residues 288–319) (Cao et al., 2019). (C) Aβ structures solved (1) for Aβ42 (Gremer et al., 2017) and Aβ40 in which all residues comprise the core (Lu et al., 2013; Kollmer et al., 2019) (2) fibrils in which the core is formed by residues 10–40 for Aβ40 (including polymorphs 2A and 3Q) (Petkova et al., 2002; Paravastu et al., 2008) and (3) for Aβ42 (core formed by residues 15–42) (Colvin et al., 2016; Wälti et al., 2016). (D) Tau fibril structures PHF and SF from Alzheimer disease patients (1) (Fitzpatrick et al., 2017) (core residues 306–378), NPF and WPF from Pick’s Disease (2) (Falcon et al., 2018) (core residues 254–378) and heparin induced structures 4R-s and 3R formed in vitro (3) (core residues 272–330) and 4R-t and 4R-j (4) (core residues 274–292, 304–321, respectively) (Zhang et al., 2019). (E) The β2m fibril core involves residues 22–85 (Iadanza et al., 2018b; Gallardo et al., 2020a). (F) The Orb2B fibril core consists of residues 176–206 (Hervas et al., 2020). (G) Human IAPP forms fibrils with residues 13–37 (Röder et al., 2020), 14–37 (Cao et al., 2020), or 13–37 (Gallardo et al., 2020a), with its early onset S20G variant adopting fibrils with two- and three filaments involving residues 15–37 in the core (Gallardo et al., 2020a). (H) PrP fibrils form fibril core with residues 170–229 revealed using cryoEM (Wang et al., 2020).