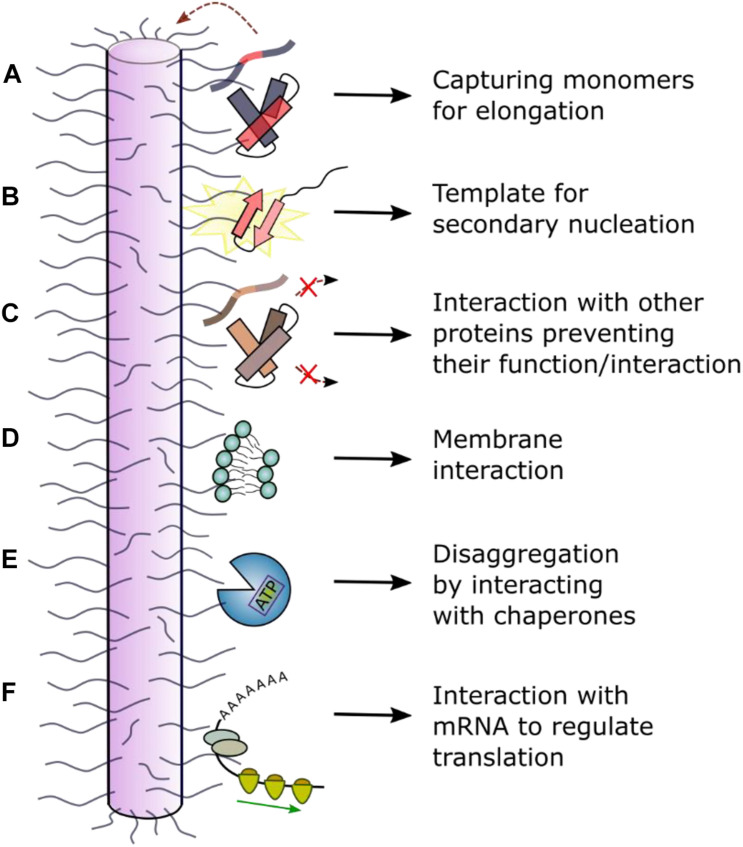
FIGURE 5.
Schematic of interactions between the dynamically disordered regions of fibrils and other molecules. Dynamically disordered regions displayed on the surface of ordered amyloid fibrils can play roles in their function and cellular dysfunction. (A) The fibril “fuzzy coat” could capture amyloid precursors and facilitate their self-assembly into amyloid by (B) secondary nucleation or other molecular events. (C) Interaction with other (non-)amyloidogenic proteins could inhibit or alter their function. (D) Interaction with membranes can result in a toxic mechanism involving membrane disruption. (E) Interaction with chaperones can result in fibril depolymerization. (F) Interaction with RNA has been observed in a functional context for the protein CPEB (cytoplasmic polyadenylation element binding) which regulates long-term memory (Khan et al., 2015; Hervas et al., 2020). In other cases, disruption of cellular RNA could enhance phase separation and lead to cellular toxicity and/or dysfunction.