Figure 3.
Drebrin Levels, Dendritic-Spine Density, and Miniature Post-synaptic Events Are Altered and Rescued by PLX3397 Treatment in Dp(16) Mice
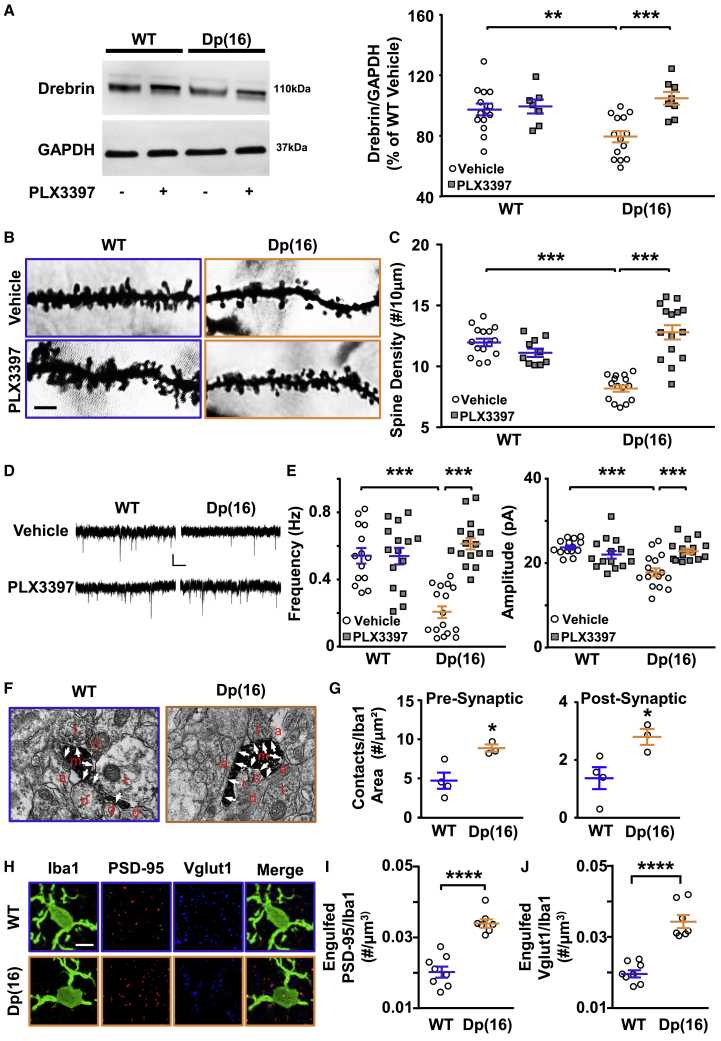
(A) Immunoblots on protein lysates of P22 WT and Dp(16) hippocampi (left). Quantification of Drebrin levels normalized to GADPH is shown (right). Bars represent the average percentage of drebrin in Dp(16) over WT hippocampi for all analyzed animals ± SEM, and symbols represent data points for each animal. ∗∗p < 0.01; ∗∗∗p < 0.001; two-way ANOVA; FInteraction (1, 39) = 7.166; p = 0.0108; Holm-Sidak post hoc test. Data were averaged across 6 independent experiments.
(B) Dendritic spines from Golgi-Cox-stained slices of hippocampi from P22 WT and Dp(16) mice treated with vehicle or PLX3397. Scale bar: 4 μm.
(C) Quantification of the spine density. Bars represent the average spine density of all analyzed cells ± SEM, and symbols represent data points for each cell (3 animals per condition). ∗∗∗p < 0.001; two-way ANOVA; FInteraction (1, 51) = 20.84; p < 0.0001; Holm-Sidak post hoc.
(D) Representative traces of mEPSCs in CA1 hippocampal pyramidal neurons from P22 WT and Dp(16) mice treated with vehicle or PLX3397. Scale bars: 10 pA and 1 s.
(E) Quantification of the mEPSCs frequency and amplitude. Bars represent the average frequency and the average amplitude for all analyzed cells ± SEM, and symbols represent data points for each cell (3 WT vehicle-treated animals and 4 animals per each remaining conditions). ∗∗∗p < 0.001; frequency: two-way ANOVA, FInteraction (2, 86) = 14.09, p < 0.0001; amplitude: two-way ANOVA, FInteraction (2, 86) = 20.61, p < 0.0001; Holm-Sidak post hoc test.
(F) Electron microscopy images of hippocampal slices from P22 WT and Dp(16) mice. Scale bar: 500 nm. Arrows point to synaptic elements; a, astrocytes; d, dendrite shaft; m, microglia; s, post-synaptic element; t, pre-synaptic element.
(G) Quantification of the interaction between microglia and pre-/post-synaptic sites. Bars represent the average number of contact points between microglia and spines of all animals analyzed ± SEM, and circles represent the average of all analyzed fields for each animal (20–44 fields/animal). (Left) ∗p < 0.05; unpaired two-tailed Student’s t test, t = 3.14, df = 5; (right) ∗p < 0.05; unpaired two-tailed Student’s t test, t = 3.09, df = 5.
(H) Confocal images of Iba1 (green), Psd-95 (red), and Vglut1 (blue)-stained hippocampal slices from P22 WT and Dp(16) animals. Scale bar: 5 μm.
(I and J) Quantification of engulfed Psd-95 or Vglut1 puncta in Iba1-labeled microglia of P22 WT and Dp(16) animals normalized to the volume of the microglia. Bars represent the average for all analyzed animals ± SEM, and symbols represent the average of all analyzed microglia for each animal (10–20 cells/animal; 1 slice per animal). ∗∗∗∗p < 0.0001; (I) two-way ANOVA; Finteraction (1, 26) = 26.23; p < 0.0001; Holm-Sidak post hoc test.
(J) Two-way ANOVA; Finteraction (1, 26) = 29.47; p < 0.0001; Holm-Sidak post hoc test.