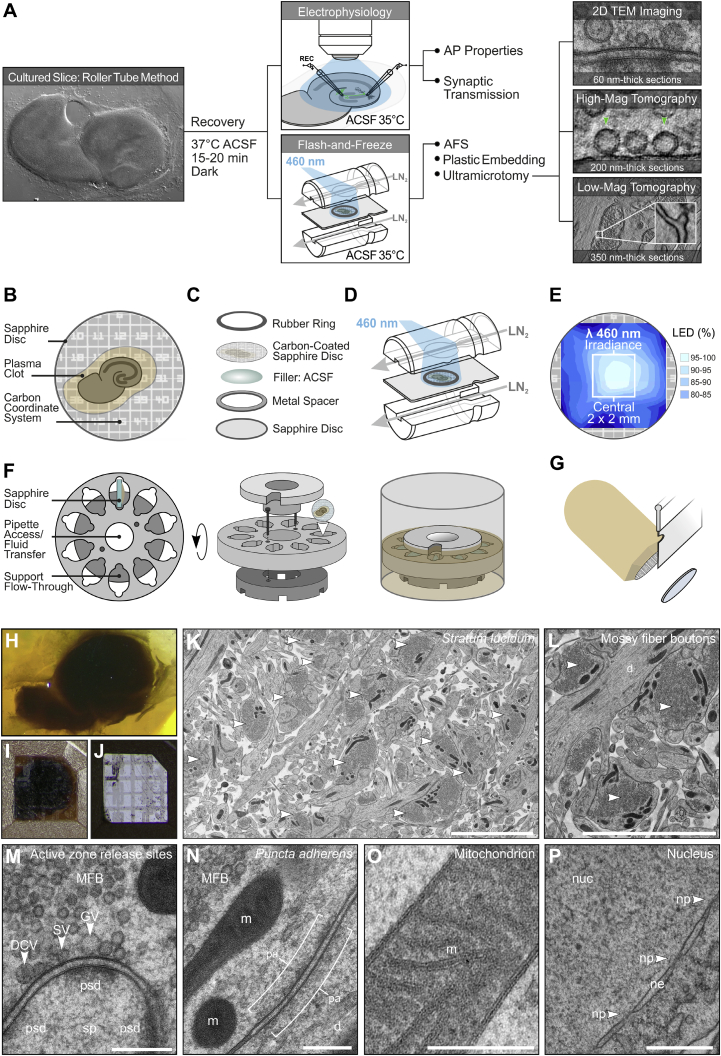
Figure 1.
Workflow for Flash-and-Freeze EM of Organotypic Hippocampal Slice Cultures
(A) Workflow for correlated electrophysiological and ultrastructural analyses.
(B) Schematic of the modified Gähwiler slice culture system.
(C) Schematic of the sapphire disc assembly.
(D) On-sapphire slices frozen in a Leica EM ICE after exposure to blue (460 nm) light.
(E) Factory calibration of the 460 nm LED illumination intensity (schematic adapted from Leica calibration data).
(F) Schematic of the configuration of a custom-built aluminum sapphire disc revolver for AFS.
(G) Carbon coordinates remain on the surface of the polymerized block following removal of the disc.
(H–J) A plastic embedded organotypic slice viewed by transmitted light before (H) and after (I) blockface trimming. The carbon used to guide trimming is visible in reflected light (J).
(K and L) Transmission electron micrographs acquired in CA3 stratum lucidum. White arrowheads, mossy fiber (MF) terminals.
(M) A high-magnification view of a MF AZ.
(N) Puncta adherens onto a dendrite.
(O and P) Vitrified samples are characterized by well-preserved mitochondrial (O) and nucleus morphology (P).
ACSF, artificial cerebrospinal fluid; AFS, automated freeze substitution; AP, action potential; d, dendrite; DCV, dense-core vesicle; GV, giant vesicle; m, mitochondrion; nuc, nucleus; ne, nuclear envelope; np, nuclear pore; pa, puncta adherens; PSD; postsynaptic density; sp, spine; SV, synaptic vesicle; TEM, transmission electron microscopy.
Scale bars: 5 μm (K and L); 200 nm (M–O); 500 nm (P).
See also Figures S1 and S2.