Table 1.
Optimization of the reaction conditions.
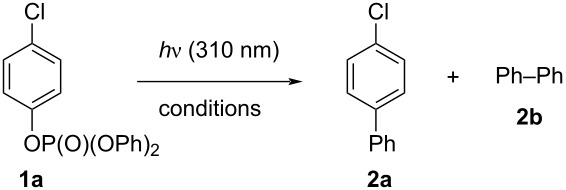 | |||
| entry | reaction conditions | λirr (nm) | products, yield (%) |
| 1 | 1a (0.02 M), CH2Cl2 | 310 | –a |
| 2 | 1a (0.02 M), CH3CN | 310 | –a |
| 3 | 1a (0.02 M), acetone | 310 | –a |
| 4 | 1a (0.02 M), CH3OH | 310 | 2a, 3b |
| 5 | 1a (0.02 M), CH3OH/H2O 2:1 | 310 | 2a, 16; 2b, 1 |
| 6 | 1a (0.01 M), CH3OH/H2O 2:1 | 310 | 2a, 6 |
| 7 | 1a (0.01 M), CH3OH/H2O 2:1 | 254 | 2a, 44 |
| 8 | 1a (0.02 M), CF3CH2OH | 310 | 2a, 45; 2b, 2 |
| 9 | 1a (0.02 M), CF3CH2OH/acetone 9:1 | 310 | 2a, 38; 2b, 2 |
| 10 | 1a (0.02 M), CF3CH2OH/acetone 4:1 | 310 | 2a, 67; 2b, 4 |
| 11 | 1a (0.02 M), CF3CH2OH/acetone 7:3 | 310 | 2a, 48; 2b, 3 |
| 12 | 1a (0.02 M), CF3CH2OH/acetone 1:1 | 310 | 2a, 14; 2b, 2 |
| 13 | 1a (0.04 M), CF3CH2OH/acetone 4:1 | 310 | 2a, 57; 2b, 2 |
| 14 | 1a (0.06 M), CF3CH2OH/acetone 4:1 | 310 | 2a, 67; 2b, 12 |
| 15 | 1a (0.02 M), CF3CH2OH/acetone 4:1c | –a | |
aNo consumption of 1a observed; b30% consumption of 1a measured; cthe reaction mixture was stored in the dark for 24 h.
