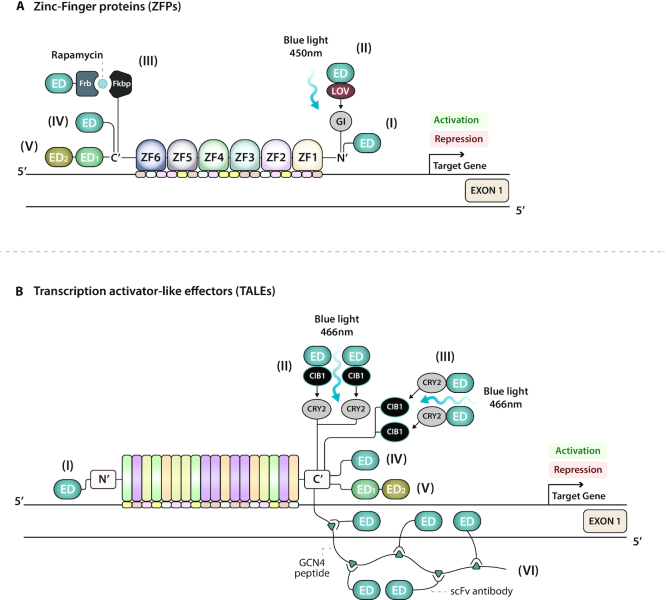
Figure 2.
Programmable DNA-targeting platforms for epigenetic editing. (A) ZFPs are artificial protein modules that bind to the major groove of DNA. Each zinc finger domain recognizes a 3-nucleotide sequence. Fusions of six ZFPs can recognize an 18-base-pair sequence. (I) A single effector domain (ED) directly fused to the N-terminus of ZFP. (II) A light-inducible system based on blue light that controls heterodimerization of the GIGANTEA (GI) protein fused to ZFP and another plant protein LOV to translocate ED to the gene of interest. (III) A chemically inducible system based on rapamycin. A fusion of ZFP to the human protein Fkbp interacts, in the presence of rapamycin, with a domain derived from human protein Frb linked to ED. This system has also been fused with CRISPR/dCas proteins (see Figure 4A-III). (IV) A single ED directly fused to the C-terminus of ZFP. (V) A bipartite ED system directly linked to the C-terminus of ZFP. (B) TALEs are highly conserved tandem repeats or monomers of 34 amino acids in length that only differ in the amino acid residues at the 12th and the 13th position. The amino acids at these two sites in each monomer target a single nucleotide in one DNA strand according to a specific code (NI = adenine, HD = cytosine, NN = guanine, and NG = thymine). Fusions of customizable modules can target an 18-base-pair sequence. (I) A single ED directly fused to the N-terminus of TALE. (II) Optogenetic modulation of gene transcription by the Light-Inducible Transcriptional Effectors (LITE) system. Blue light triggers the interaction between TALE fused to the plant light-sensitive cryptochrome 2 (CRY2) protein and its interacting partner CIB1 linked to ED. (III) A spatiotemporal light-inducible system based on an inverted heterodimerizing fusion protein approach. This system has also been fused with CRISPR/dCas9 (see Figure 4A-I). (IV) A single ED directly fused to the C-terminus of TALE. (V) A bipartite ED system directly linked to the C-terminus of TALE. (VI) The SunTag system C-terminally fused to TALE. This technology involves a protruding GCN4 peptide that contains several antibody-binding sites (triangles) that can recruit multiple single-chain antibodies (scFv) fused to EDs for amplification of epigenetic editing activity. The system has also been devised with CRISPR/dCas proteins (see Figures 3A-V and 4A-IV).