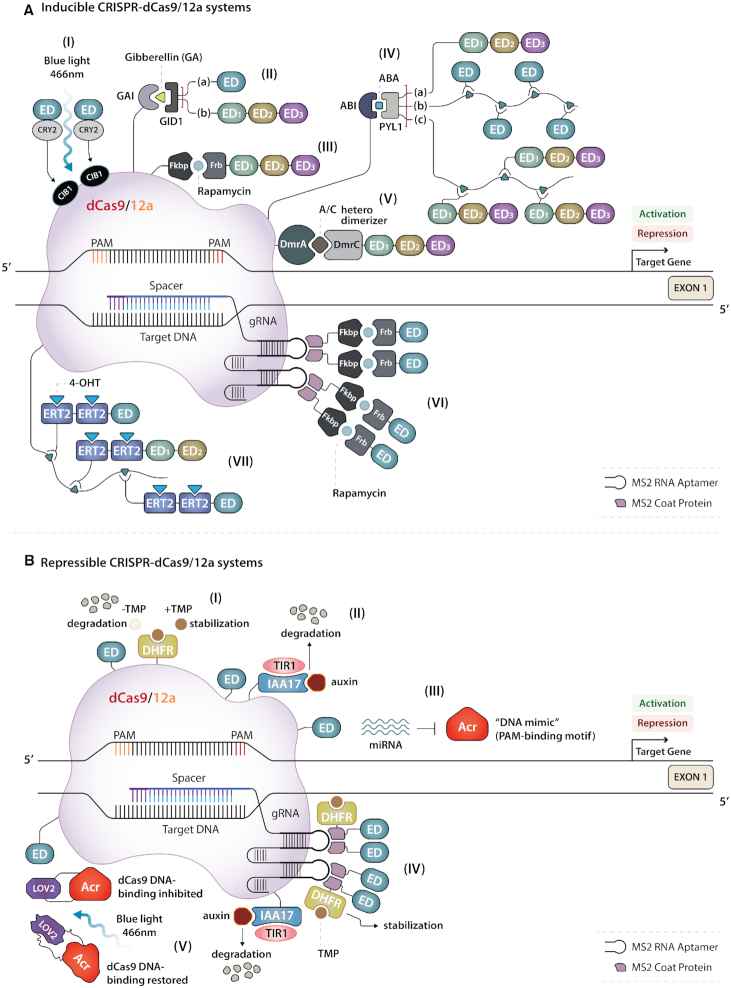
Figure 4.
Inducible and repressible systems for precise and dynamic control of CRISPR-dCas9- and 12a-mediated epigenetic editing. (A) (I) The light-activated CRISPR-dCas9 effector (LACE) system induces spatiotemporal gene regulation based on exposure to blue light. (II) The chemical gibberellin (GA) induces dimerization of dCas fused to the GA-insensitive (GAI) plant protein and its binding partner gibberellin-insensitive dwarf1 (GID1) linked to either (a) single EDs or (b) tripartite ED systems. (III) The Fkbp-Frb technology recruits tripartite ED systems in the presence of rapamycin. (IV) The chemical abscisic acid (ABA)-inducible system. ABA triggers dimerization of dCas fused to the ABA-insensitive 1 (ABI) plant protein and its PYL1 interacting domain directly linked to (a) tripartite ED systems or to the SunTag system, which recruits (b) single EDs or (c) tripartite ED systems. (V) A drug-dependent system based on DmrA and DmrC domains fused to dCas and EDs, respectively, which interact only in the presence of the rapamycin analog A/C heterodimerizer. (VI) The Fkbp/Frb inducible recruitment for epigenome editing (FIRE) system combines the gRNA MS2 technology with the rapamycin-dependent dimerization approach. (VII) The hybrid drug inducible technology (HIT) based on the SunTag system. scFv antibodies are engineered with two copies of a mutated human estrogen receptor (ERT2) followed by single and multiple EDs. 4-Hydroxytamoxifen (4-OHT) induces nuclear translocation of these constructs that are otherwise retained in the cytoplasm. (B) (I) A drug-tunable system for conditional stabilization of dCas linked to ED (dCas9-ED) and N-terminally fused to a dihydrofolate reductase (DHFR)-derived destabilization domain. The addition of trimethoprim (TMP) temporally stabilizes the fusion construct. (II) Auxin-Inducible Degron technology (AID) involves tagging dCas-ED with the auxin plant hormone-sensitive domain IAA17 and co-expression of the auxiliary protein TIR1. The addition of auxin targets the chimeric dCas-ED protein for rapid proteasomal degradation. (III) Anti-CRISPR (Acr) proteins, e.g., AcrIIA4, interfere, and compete with dCas9-ED DNA recognition. The microRNA (miRNA)-responsive ‘Acr switch’ system is designed to cell-specifically regulate epigenetic editing. High levels of a specific miRNA can block Acr expression, thereby releasing dCas9-ED. (IV) A combinatorial strategy that couples the DHFR-TMP system, based on the MS2 ED-recruitment approach, with the AID technology. TMP results in stability, which can be abrogated by the addition of auxin to the system. (V) Optogenetic control based on the CASANOVA system. Acr fused to the plant photosensor LOV2 constitutively interferes with dCas9-ED DNA targeting. Blue light unfolds and impairs Acr-LOV2 fusion, thereby releasing dCas9-ED.