Fig. 4.
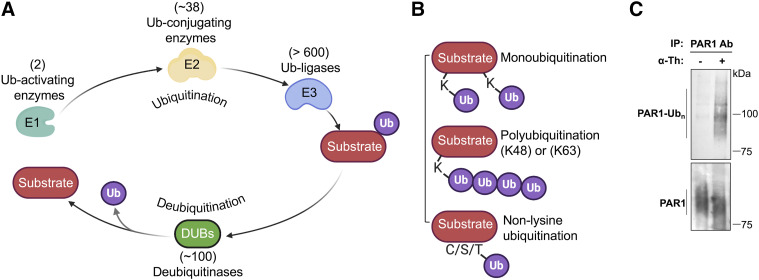
Ubiquitin modifying enzymes, ubiquitin linkages and detection. (A) Ubiquitination of substrate proteins is carried out sequentially by a ubiquitin (Ub)–activating enzyme E1, ∼38 ubiquitin-conjugating enzyme E2s, and >600 ubiquitin ligase E3 enzymes. Ubiquitin is enzymatically cleaved by ∼100 deubiquitinases to release ubiquitin back to cytosolic pool. (B) GPCRs are modified with different ubiquitin conjugations, including monoubiquitin (single or multiple monoubiquitin) and K48- or K63-linked polyubiquitin, which regulate distinct functions. Nonlysine ubiquitination has also been reported to occur on GPCRs. (C) Ubiquitination of endogenous PAR1 ubiquitination in endothelial cells after 7-minute stimulation with 10 nM thrombin (α-Th) detected by immunoblotting of immunoprecipitated (IP) PAR1 using anti-pan ubiquitin P4D1 antibody that detects multiple Ubn species. N-terminal proteolytic cleavage of PAR1 by thrombin results in reduced protein size of total protein detected by immunoblotting with PAR1 antibody (ab), bottom panel.