Fig. 1.
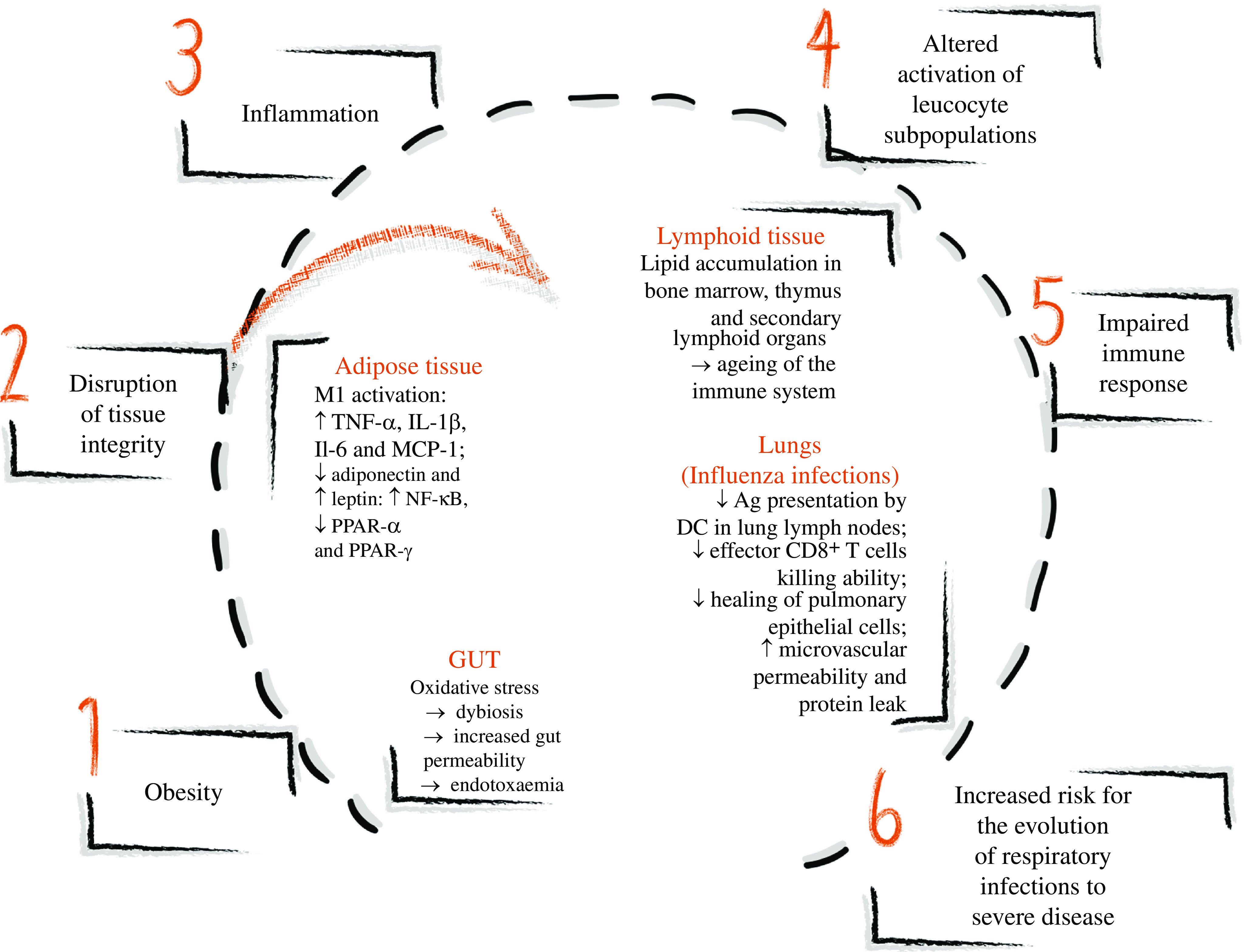
Obesity and immune alterations in different tissues leading to impaired immune response and increased risk for the evolution of respiratory infectious to severe disease. Obesity determines the stress and disruption of many tissues’ integrity leading to inflammation. In the adipose tissue, the enlarging adipocytes present oxidative stress and increase the release of NEFA in the adipose tissue, activating classical macrophages (M1), which produce IL-1β, TNF-α and IL-6. An unfavourable hormone milieu also promotes inflammation: low adiponectin and high leptin productions are observed in obesity. In the lymphoid tissue, lipid accumulation occurs in the bone marrow, thymus and secondary lymphoid organs, altering the immune tissue architecture similarly to findings observed in ageing. In the gut, oxidative stress causes dysbiosis, increasing gut permeability and inducing endotoxaemia, characterised by an increase in gut-derived plasma lipopolysaccharide, which induces inflammation. In the lungs, experimental diet-induced obesity studies of influenza infections showed the reduced ability of dendritic cells (DC) to present antigens (Ag) to T cells, impairing monocyte and CD8+ T cell recruitment and reducing IL-2 and IL-12 production. Effector CD8+ T cells demonstrated a lower ability to kill influenza-infected cells, and the healing of pulmonary epithelial cells is compromised, resulting in microvascular permeability and protein leak. Overall, the inflammation induced by obesity causes altered activation of leucocyte subpopulations, impairing the immune response and increasing the risk of the evolution of respiratory infection to severe disease. ↑, Increase; ↓, decrease.
