Figure 2.
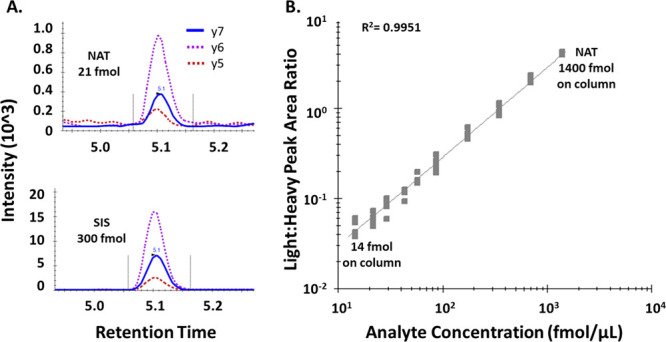
Validation of bevacizumab LC-MRM assay. (A) Two transitions of the peptide STAYLQM(ox)NSLR ((M + 2H)2+ → y6+, (M + 2H)2+ → y5+) were used as qualifiers and one transition ((M+2H)2+ → y7+) was used as the quantifier. (B) Full STAYLQM(ox)NSLR calibration curve. NAT peptide was spiked at different concentrations into digested plasma, using SIS as constant normalizer, followed by H2O2 oxidation and LC-MRM. The linear range was from 14 to 1400 fmol NAT on-column. The curve was conducted in 5 independent replicates, yielding coefficients of variations <15.6% for all points except 14.6 fmol with a CV of 20.8% (Table 1).
