Abstract
Purpose:
Inflammatory breast cancer (IBC) rates increased in the United States before the turn of the 21st century. We examine trends by estrogen-receptor (ER) status since then.
Methods:
Using data from the Surveillance, Epidemiology and End Results (SEER) program for years 2001-2015, we calculated age-adjusted incidence rates for IBC (defined by AJCC TNM category T4d, extent of disease codes, and morphology code 8530) by ER status, which was imputed if unknown, among women aged 25-84 years. For comparison, we included other locally advanced breast cancer and other breast cancers partitioned into localized and regional/distant/unstaged. We fit joinpoint log-linear models to annual rates to calculate annual percentage change (APC) and average annual percentage change (AAPC).
Results:
Slight increases in ER+ IBC rates among women aged 25-44 (AAPC = 0.5) were similar to other advanced tumor types, but declines among women aged 45-84 (AAPC=−2.2) were greater. Declines in ER− IBC rates for women aged 25-84 (AAPC=−3.7) were greater than for other tumor types.
Conclusions:
Our results show a reversal of the rising rates of IBC overall reported at the end of the 20th century. Direction of trends for IBC are consistent with other breast cancer types, except for ER+ localized breast cancer in older women. Decreasing parity and rising prevalence of older age at first birth may contribute to declining rates of ER− IBC. Otherwise, patterns of changing risk factors are inconsistent with the trends we observed. Further studies of IBC are necessary to identify additional risk factors and possible preventive strategies.
Keywords: inflammatory breast cancer, trends, incidence rates, SEER
Introduction
Inflammatory breast cancer (IBC) is an aggressive type of locally advanced breast cancer (LABC) characterized by erythema, edema, and peau d’orange of the breast, often with no underlying tumor mass [1]. A pathologic diagnosis of cancer in the breast parenchyma or dermal lymphatics is also required for diagnosis [2]. The signs characteristic of IBC generally arise quickly in the affected breast. This differentiates IBC from neglected LABC, which is a distinct clinicopathologic entity [3] where the skin changes present late in the course of the disease. IBC constitutes approximately 2% of breast cancer cases in the United States, but it accounts for 7% of breast cancer deaths [4].
Most previous analyses using the National Cancer Institute’s (NCI) Surveillance, Epidemiology and End Results (SEER) program data showed increases in IBC incidence over time for several decades before the turn of the 21st century [1, 4–5]. One analysis of IBC incidence from 1992-2009 did not report increasing trends, but it was unclear whether this analysis properly defined IBC over time [6].
Trends for estrogen receptor (ER)-positive (+) and ER-negative (−) IBC have not been examined. Notably, IBC has a higher proportion of ER− breast cancer than breast cancer as a whole [1]. Total breast cancer rates by tumor ER status have diverged since 2000. The declines in ER+ breast cancer during 2000-2004 are generally thought to be due to decreases in use of hormone replacement therapy among menopausal women [7]. Rates for ER+ breast cancer have been increasing at least since 2004, while rates for ER− breast cancer have been declining [7]. Here we assess overall rates and temporal trends in IBC by ER status during 2001-2015 with imputation of missing data on ER status. For comparison, we include other types of LABC, a major differential diagnosis for IBC [8], as well as other breast cancers partitioned into localized and regional/distant/unstaged stages.
Methods
Data Source
We used cancer incidence data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) 18 registries for cases diagnosed during the years 2001-2015. The 18 registries are: Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San-Francisco-Oakland, Seattle-Puget Sound, Utah, Los Angeles, San Jose-Monterey, Rural Georgia, Alaska Native Tumor Registry, Greater California, Greater Georgia, Kentucky, Louisiana, and New Jersey. Data for Louisiana from July, 2005 to December, 2005 were excluded to adjust for the impact of Hurricanes Katrina and Rita. Per the 2010 Census, the SEER 18 registries cover 27.8% of the United States population.
We limited our analysis to 2001-2015 because trends in IBC for earlier periods have already been reported; moreover, we determined that the degree of missing data on ER status before this period was so substantial as to make analyses by ER status unreliable (e.g. 43.8% of IBC cases had missing ER status in 1990). Finally, due to the relatively small number of IBC cases, we concluded it was best to plot 10-year age-specific rates by 5-year time periods (2001-2005, 2006-2010, 2011-2015).
Case definitions:
We identified first female breast cancer cases (ICD-O-3/WHO 2008; SEER site recode = ‘Breast’) in the 18 registries aged 25-84 years diagnosed during January 1, 2001 to December 31, 2015. Cases were classified as IBC if they had at least one of the morphology, stage, or extent of disease criteria described in Table 1. Non-IBC cases were defined as other LABC if they met the LABC stage criteria shown in Table 1. We defined all remaining cases as localized or non-localized other breast cancer using SEER historic stage A. This resulted in four mutually exclusive groups: IBC (N =11,438), other LABC (N =12,876), and localized (N =490,534) and non-localized=regional/distant/unstaged (N = 272,708) other breast cancer.
Table 1.
Definition of Breast Cancer Cases
| Case Type | Definition |
|---|---|
| Inflammatory breast cancer (IBC) |
Morphology: 2001-2015 • ICD-O-3 (converted from ICD-O-2 for 1988-2000) = 8530 “Inflammatory Carcinoma” or Stage: 2001-2015 • AJCC TNM, T = T4d “Inflammatory Carcinoma” or Extent of Disease: 2001-2003 (Historic) • 70: “Inflammatory carcinoma, including diffuse (beyond that directly overlying the tumor) dermal lymphatic permeation or infiltration.” 2004-2010 (Collaborative Staging) • 71: “Diagnosis of inflammatory carcinoma WITH a clinical description of inflammation, erythema, edema, peau d’ orange, etc., involving not more than 50% of the skin of the breast, or percent of involvement not stated, WITH or WITHOUT dermal lymphatic infiltration. Inflammatory carcinoma, NOS.” • 73: “Diagnosis of inflammatory carcinoma WITH a clinical description of inflammation, erythema, edema, peau d’orange, etc., of more than 50% of the breast, WITH or WITHOUT dermal lymphatic infiltration.” 2011-2015 (Collaborative Staging) • 600: “Diagnosis of inflammatory carcinoma WITH a clinical description of inflammation, erythema, edema, peau d’orange, etc., involving less than one-third (33%) of the skin of the breast, WITH or WITHOUT dermal lymphatic infiltration.” • 725: “Diagnosis of inflammatory carcinoma WITH a clinical description of inflammation, erythema, edema, peau d’orange, etc., involving one-third (33%) or more but less than or equal to one-half (50%) of the skin of the breast, WITH or WITHOUT dermal lymphatic infiltration.” • 730: “Diagnosis of inflammatory carcinoma WITH a clinical description of inflammation, erythema, edema, peau d’orange, etc., involving more than one-half (50%) of the skin of the breast, WITH or WITHOUT dermal lymphatic infiltration.” • 750: “Diagnosis of inflammatory carcinoma WITH a clinical description of inflammation, erythema, edema, peau d’orange, etc., but percent of involvement not stated, WITH or WITHOUT dermal lymphatic infiltration. Diagnosis of inflammatory carcinoma WITHOUT a clinical description of inflammation, erythema, edema, peau d’orange, etc., WITH or WITHOUT dermal lymphatic infiltration. Inflammatory carcinoma, NOS.” • 780: “Stated as T4d with no other information on extension.” |
| Non-inflammatory locally advanced breast cancer (LABC) |
Stage: 2001-2015 Not an IBC case as defined above and: • AJCC TNM, T = T4a (extension to the chest wall, not including only pectoralis muscle adherence/invasion), or • AJCC TNM, T = T4b (ulceration and/or ipsilateral satellite nodules and/or edema (including peau d’orange) of the skin, which do not meet the criteria for inflammatory carcinoma), or • AJCC TNM, T = T4c (both T4a and T4b) |
| Other localized breast cancer | SEER Historic Stage A = “localized” |
| Other regional/distant/unstaged other breast cancer | SEER historic stage A = “regional/distant/unstaged” |
Criteria and codes used to identify IBC in SEER have changed over time. From 1990-2003, the percentage of the breast with signs indicative of IBC was not specified using either AJCC stage (T4d) or SEER extent of disease (EOD) codes [9–13, 15]; from 2004-2009 with Collaborative Staging version 1, the clinical findings were required to involve the majority of the skin of the breast [2, 16], and from 2010-2015 with Collaborative Staging version 2 to involve a third or more of the breast [14, 16]. Furthermore, starting in 2007 the use of morphology code 8530 required an indication of inflammatory breast cancer on the pathology report [17, 18]. However, using other EOD codes, we were able to define IBC for the entire study period as it was defined between 1990-2003.
We stratified cases according to ER+ and ER− status. The categories of Extent of Disease - CS ER Status Recode Breast Cancer (1990+) are: positive, negative, borderline, unknown [19]. Clinicians have traditionally used a cutoff of ≥10% stained cells as positive, 0% as negative, and 1-9% as borderline [20]. In 2010, new guidelines for interpreting test results indicated that the code for borderline ER status should rarely, if ever, be used; any test which resulted in at least 1% of the cells staining positive is a positive test and <1% of cells stained is a negative test [21]. Therefore, we included borderline ER status as ER+. For analyses using all races combined we imputed ER status (positive or negative) for each tumor type (IBC, LABC, other localized, other non-localized) for those with unknown categories according to the proportion of known ER+ and ER− breast cancers by year of diagnosis and 10-year age group (25-34, 35-44, 45-54, 55-64, 65-74, 75-84) [22]. For analyses by race/ethnicity (white non-Hispanic, white Hispanic, Black, Asian/Pacific Islander), we imputed ER status by year of diagnosis using age groups 25-84 combined.
Overall, 10.0% of IBC cases, 8.5% of LABC cases, 6.6% of localized cases, and 10.1% of other non-localized cases had /unknown ER status, but this percentage fell over time. In 2001, the percentages of IBC cases, LABC cases, localized and non-localized other breast cancer cases with unknown ER status were 23.7%, 23.4%, 18.3%, and 20.7%, respectively. In 2003, information on ER status began to be collected under Collaborative Staging version 1 and was required by the American College of Surgeons Commission on Cancer approved hospitals [23]; thereafter, from 2004 to 2015 the percentage of IBC, LABC, localized and non-localized other breast cancer cases with unknown ER status fell from 13.8% to 3.7%, from 11.4% to 3.8%, from 9.4% to 1.9%, and from 12.9% to 5.4%, respectively.
Statistical Analyses
We calculated age-adjusted incidence rates for all races combined and by race/ethnicity by tumor type and ER status for women ages 25-84 for the years 2001-2015 based on the 2000 U.S. standard population using SEER*Stat software package version 8.1.5. We also calculated age-adjusted incidence rates in 10-year age groups and three time-periods (2001-2005, 2006-2010, 2011-2015). In addition, we calculated annual age-adjusted incidence rates. We reported rates as cases per 100,000 woman-years; we did not present any rates in tables or figures based on fewer than 16 cases (see Supplemental Table 1 for counts and rates by single year of diagnosis and 10-year age group).
We fit joinpoint log-linear models to annual overall and age-group specific age-adjusted rates for each tumor type over time (2001-2015) using the Joinpoint Regression Program, Version 4.5.0.1 - June 2017; Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute. This software initially fits a straight line (0 knot points or joinpoints), then iteratively uses permutation tests [24] to assess whether adding joinpoints for subsequently segmented lines better describes the data, identifies the year(s) when the slopes of the lines at the joinpoints are statistically significantly different (two-sided p value < 0.05) and obtains the slope of the segmental lines (annual percentage change (APC)). We set the minimum number of observations for each joinpoint segment as 4, and we allowed up to three segments. We then calculated average annual percentage changes (AAPC) to summarize and compare trends for different groups that had different joinpoints and thus different time partitions over which they had a constant rate of change. The AAPC reduces to the APC if the rate of change is constant over the entire time period. We did not adjust 95% confidence intervals (CI) for the imputation of ER status.
We plotted age-adjusted incidence rates by tumor type and ER status for 10-year age groups and 5-year diagnosis time periods on a log y and linear x scale, such that a rate change of 1% per year was portrayed by an angle of 10 degrees [25].
Results
The overall age-adjusted incidence rates per 100,000 women aged 25-84 years during 2001-2015 for ER+ and ER− IBC were 1.41 and 1.23, respectively. The corresponding incidence rates for LABC, localized breast cancer, and other non-localized breast cancer were 2.02 and 0.96, 92.49 and 20.20, and 49.53 and 13.87, respectively.
Compared to non-Hispanic whites, rates of ER+ and ER− IBC were higher in blacks, were similar in Hispanic whites, and were lower in Asian/Pacific Islanders (Table 2). Blacks also had higher rates of ER+ and ER− LABC, and ER− other localized and non-localized breast cancer.
Table 2.
Incidence rates per 100,000 during 2001-15 for inflammatory breast cancer (IBC), other locally advanced breast cancer (LABC), other localized breast cancer (OLBC) and other non-localized breast cancer (ONLBC) by estrogen receptor (ER)-positive (+) and negative (−) status and race/ethnicity.
| Non-Hispanic White | Hispanic White | Black | Asian/Pacific Islander | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ER status | Tumor type | Count | Rate | Count | Rate | Count | Rate | Count | Rate |
| ER + | IBC | 4048 | 1.44 | 786 | 1.38 | 903 | 1.88 | 313 | 0.73 |
| ER + | LABC | 5746 | 1.97 | 907 | 1.76 | 1279 | 2.78 | 739 | 1.76 |
| ER + | OLBC | 302487 | 104.53 | 33502 | 64.12 | 31325 | 67.43 | 31845 | 75.61 |
| ER + | ONLBC | 148692 | 53.03 | 22830 | 40.94 | 23155 | 48.78 | 15989 | 37.51 |
| ER − | IBC | 3296 | 1.19 | 659 | 1.13 | 1041 | 2.14 | 285 | 0.66 |
| ER − | LABC | 22551 | 0.80 | 489 | 0.88 | 1028 | 2.15 | 315 | 0.74 |
| ER − | OLBC | 56889 | 20.36 | 8860 | 15.66 | 13750 | 28.56 | 6592 | 15.39 |
| ER − | ONLBC | 35269 | 12.92 | 7144 | 12.29 | 11704 | 24.19 | 4415 | 10.30 |
The racial/ethnic composition of the SEER 18 population changed over the study period. The percent non-Hispanic whites declined from 64.6% in 2001 to 56.3% in 2015, Hispanic whites increased from 13.8% to 17.7%, blacks rose from 11.6% to 12.5%, Asian/Pacific Islanders rose from 8.9% to 12.0% and American Indian/Alaskan natives remained fairly constant at 1.1% and 1.5%.
Plots of the age-adjusted incidence rates for 10-year age groups by 5-year diagnosis time periods for all race/ethnicities combined are shown in Figure 1 (counts are shown in Supplemental Table 2). Numbers of cases by age and time period were insufficient to allow this analysis by race/ethnicity. In general, rates of ER+ IBC, LABC, and other non-localized breast cancer increased for ages 25-44 and decreased for ages 45-84. ER+ rates of other localized breast cancer increased for most age groups. Rates of ER− IBC, LABC, other localized breast cancer and other non-localized breast cancer declined in all age groups.
Figure 1.
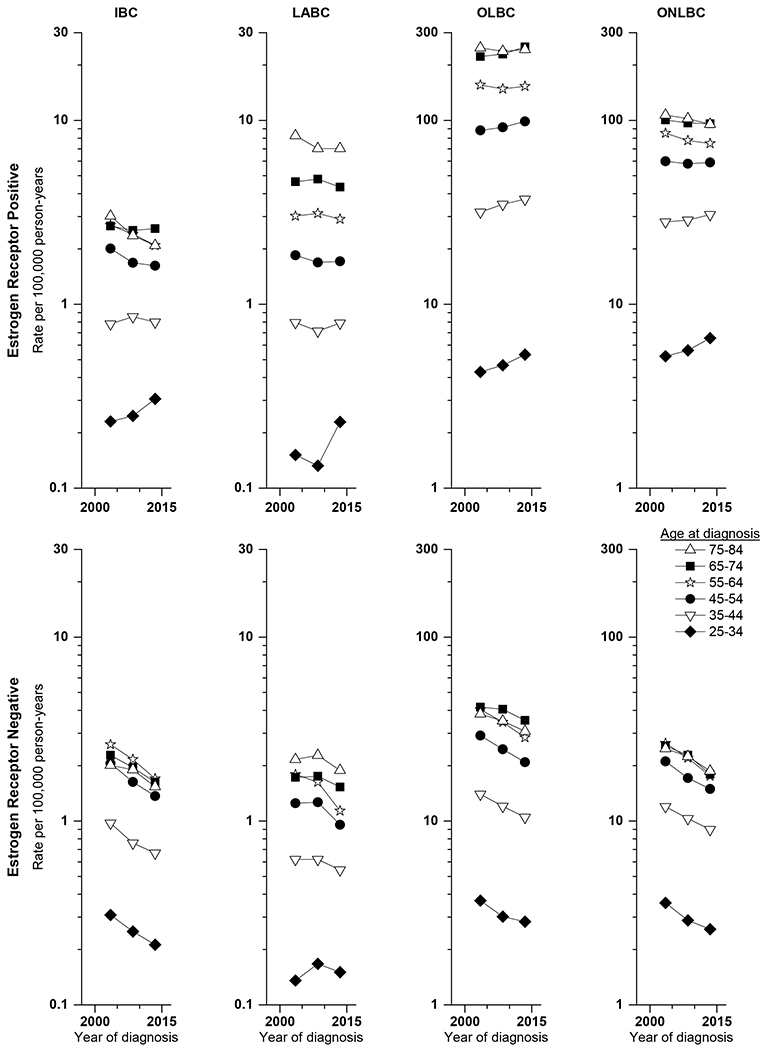
Age-adjusted incidence rates for 10-year age groups by 5-year diagnosis time periods for inflammatory breast cancer (IBC), other locally advanced breast cancer (LABC), other localized breast cancer (OLBC), and other non-localized breast cancer (ONLBC) by estrogen receptor status.
AAPCs and APCs from joinpoint analyses are presented by tumor type for ages 25-44 and 45-84 for ER+ disease and for ages 25-84 for ER− disease in Table 3. The AAPC for ER+ IBC among younger women was approximately the same as for LABC and other non-localized breast cancer, but the slight increase was not statistically significantly different from 0. Declines in rates among older women were more rapid, particularly during years 2001-05. The most rapid declines for other tumor types, except for LABC, were also evident during the years 2001 to 2004. Decreases in rates of ER− IBC overall were somewhat more rapid than for other tumor types.
Table 3.
Annual percent change (APC) and average annual percent change (AAPC) in rates from joinpoint analyses during 2001-2015 of estrogen receptor (ER)-positive and ER-negative inflammatory breast cancer (IBC), other locally advanced breast cancer (LABC), other localized breast cancer (OLBC) and other non-localized breast cancer (ONLBC) for broad age groups.
| AAPC | JP Trend 1 | JP Trend 2 | JP Trend 3 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Type | # Cases | 2001-15 | Years | APC | Years | APC | Years | APC | |||||||
| ER+ | 25-44 | IBC | 963 | 0.5 | 2001-15 | 0.5 | ||||||||||
| 25-44 | LABC | 844 | 0.6 | 2001-15 | 0.6 | |||||||||||
| 25-44 | OLBC | 35464 | 1.2 | * | 2001-15 | 1.2 | * | |||||||||
| 25-44 | ONLBC | 31455 | 0.6 | * | 2001-15 | 0.6 | * | |||||||||
| 45-84 | IBC | 5123 | −2.2 | * | 2001-05 | −6.0 | * | 2005-15 | −0.7 | |||||||
| 45-84 | LABC | 7881 | −1.1 | * | 2001-15 | −1.1 | * | |||||||||
| 45-84 | OLBC | 366292 | 0.0 | 2001-04 | −5.1 | * | 2004-15 | 1.4 | * | |||||||
| 45-84 | ONLBC | 181433 | −1.1 | * | 2001-04 | −4.7 | * | 2004-15 | −0.1 | |||||||
| ER− | 25-84 | IBC | 5305 | −3.7 | * | 2001-15 | −3.7 | * | ||||||||
| 25-84 | LABC | 4105 | −0.8 | 2001-06 | 4.1 | 2006-12 | −6.5 | * | 2012-15 | 2.9 | ||||||
| 25-84 | OLBC | 86946 | −2.1 | * | 2001-05 | −0.1 | 2005-12 | −4.0 | * | 2012-15 | 0.0 | |||||
| 25-84 | ONLBC | 59095 | −2.7 | * | 2001-07 | −2.2 | * | 2007-12 | −5.3 | * | 2012-15 | 0.6 | ||||
Indicates 95% confidence interval excludes 0.0.
AAPCs and APCs from joinpoint analyses by tumor type, ER status and race/ethnicity are shown in Table 4. We do not present results by race/ethnicity for ER+ tumor types among those aged 25-44 because of small numbers of cases. Among women age 45-84, the overall decline in rates of ER+ IBC shown in Table 3 was evident for all racial/ethnic groups, but declines were less rapid and not statistically significant for blacks and Asian/Pacific Islanders. The overall decline in rates of ER+ LABC and other non-localized breast cancer (Table 3) were largely attributable to declines in non-Hispanic and Hispanic whites. The absence of an average change in rates in other localized breast cancer (Table 3) masked a slight and not statistically significant average decline in rates among non-Hispanic whites and increases in the other racial/ethnic groups. Rates of all four types of ER− breast cancer for ages 25-84 combined declined among all racial/ethnic groups, but not always statistically significantly for LABC and other non-localized breast cancer.
Table 4.
Annual percent change (APC) and average annual percent change (AAPC) in rates from joinpoint analyses of estrogen receptor (ER)-positive and ER-negative inflammatory breast cancer (IBC), other locally advanced breast cancer (LABC), other localized breast cancer (OLBC), and other non-localized breast cancer (ONLBC) from joinpoint analyses for broad age groups by race/ethnicity: non-Hispanic white (NHW), Hispanic white (HW), Black, Asian/Pacific Islander (PI)
| AAPC | JP Trend 1 | JP Trend 2 | JP Trend 3 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Type | Race/ethnicity | # Cases | 2001-15 | Years | APC | Years | APC | Years | APC | ||||||||
| ER+ | 45-84 | IBC | NHW | 3561 | −2.5 | * | 2001-04 | −8.5 | * | 2004-15 | −0.8 | |||||||
| HW | 577 | −2.7 | * | 2001-15 | −2.7 | * | ||||||||||||
| Black | 718 | −1.5 | 2001-15 | −1.5 | ||||||||||||||
| Asian/PI | 234 | −0.7 | 2001-15 | −0.7 | ||||||||||||||
| LABC | NHW | 5358 | −1.1 | * | 2001-15 | −1.1 | * | |||||||||||
| HW | 756 | −1.3 | 2001-15 | −1.3 | ||||||||||||||
| Black | 1096 | −0.1 | 2001-15 | −0.1 | ||||||||||||||
| Asian/PI | 640 | 0.1 | 2001-15 | 0.1 | ||||||||||||||
| OLBC | NHW | 280766 | −0.1 | 2001-04 | −4.6 | * | 2004-15 | 1.2 | * | |||||||||
| HW | 28660 | 1.6 | * | 2001-15 | 1.6 | * | ||||||||||||
| Black | 27618 | 2.2 | * | 2001-15 | 2.2 | * | ||||||||||||
| Asian/PI | 27324 | 1.1 | * | 2001-04 | −3.2 | * | 2004-15 | 2.4 | * | |||||||||
| ONLBC | NHW | 130702 | −1.5 | * | 2001-04 | −5.3 | * | 2004-09 | 0.3 | 2009-15 | −1.0 | * | ||||||
| HW | 17508 | −0.8 | 2001-04 | −6.1 | * | 2004-11 | 2.0 | * | 2011-15 | −1.4 | ||||||||
| Black | 19039 | 0.2 | 2001-05 | −2.1 | 2005-09 | 3.1 | 2009-15 | −0.2 | ||||||||||
| Asian/PI | 12544 | 0.4 | 2001-15 | 0.4 | ||||||||||||||
| ER− | 25-84 | IBC | NHW | 3296 | −3.9 | * | 2001-15 | −3.9 | * | |||||||||
| HW | 659 | −3.5 | * | 2001-15 | −3.5 | * | ||||||||||||
| Black | 1041 | −3.5 | * | 2001-15 | −3.5 | * | ||||||||||||
| Asian/PI | 285 | −4.7 | * | 2001-15 | −4.7 | * | ||||||||||||
| LABC | NHW | 2251 | −0.8 | 2001-06 | 4.8 | 2006-11 | −7.7 | 2011-15 | 1.3 | |||||||||
| HW | 489 | −2.4 | * | 2001-15 | −2.4 | * | ||||||||||||
| Black | 1028 | −3.4 | * | 2001-15 | −3.4 | * | ||||||||||||
| Asian/PI | 315 | −3.1 | 2001-15 | −3.1 | ||||||||||||||
| OLBC | NHW | 56889 | −2.6 | * | 2001-04 | −0.1 | 2004-15 | −3.3 | * | |||||||||
| HW | 8860 | −1.7 | * | 2001-05 | 1.7 | 2005-12 | −5.0 | * | 2012-15 | 1.5 | ||||||||
| Black | 13750 | −1.9 | * | 2001-15 | −1.9 | * | ||||||||||||
| Asian/PI | 6592 | −2.4 | * | 2001-15 | −2.4 | * | ||||||||||||
| ONLBC | NHW | 35269 | −3.3 | * | 2001-07 | −3.0 | 2007-11 | −5.8 | * | 2011-15 | −1.3 | |||||||
| HW | 7144 | −1.8 | 2001-05 | 2.4 | 2005-2012 | −5.7 | * | 2012-15 | 2.0 | |||||||||
| Black | 11704 | −3.2 | * | 2001-15 | −3.2 | * | ||||||||||||
| Asian/PI | 4415 | −2.6 | * | 2001-15 | −2.6 | * | ||||||||||||
Indicates 95% confidence interval excludes 0.0.
Discussion
Based on SEER data that include nearly 28% of the U.S. population, rates of both ER+ and ER− IBC have declined since the turn of the 21st century, except for ER+ tumors among women younger than age 45. Declines in ER-positive IBC among women aged 45-84 were greater for non-Hispanic and Hispanic whites than for Blacks and Asian/ Pacific Islanders, while declines in ER-negative IBC were similar across racial/ethnicity groups. This is a reversal of the rising rates of IBC overall at the end of the 20th century [1,4–5]. The direction of trends for ER+ IBC was the same as for other advanced breast cancer types over the period 2001-2015; however, differences were evident with other localized breast cancer, where rates of ER+ breast cancer among older women generally increased, particularly among races/ethnicities other than non-Hispanic whites. The direction of trends for ER− IBC was the same as for all other tumor types.
We are not aware of other reports of trends in IBC rates according to ER status. Our results as a whole are generally consistent with a report also based on SEER 18 for all breast cancer using imputed missing ER status which showed significant increases in rates of ER+ breast cancer overall from 2000-2009 among women younger than 50 and declines among older women from 2000 followed by increases starting in the mid-2000s. Notably, the rates in 2009 among older non-Hispanic whites (the vast majority of women) remained lower than those in 2000 [7]. Decreases in risk of ER− breast cancer from 2000-2009 were evident in most age and ethnic groups [7].
Several methodologic issues could influence interpretation of our results. The imputation method we used is based on a formal imputation model, albeit a simple one (22); however, this method underestimates the variance of the estimates. Thus, the results we report as statistically significant might not be so if the extra variance from the imputation had been accounted for, but those reported as not statistically significant would remain so. The changing definitions of IBC and availability of estrogen receptor status determination also need to be considered. Using imputed ER status for those with unknown ER values, we did not find major shifts in trends for both ER+ and ER− IBC around 2003/2004 when information on ER status began to be collected under Collaborative Staging version 1 and was required by the American College of Surgeons Commission on Cancer approved hospitals [23]. However, there was a joinpoint in 2005 for ER+ IBC among women aged 45-84, similar to joinpoints in 2004 for other localized breast cancer and other non-localized breast cancer. Changes in incidence rates of total breast cancer in the early 2000s have been attributed at least in part to changes in menopausal hormone use [26], which has not been well-studied in relationship to risk of IBC. There were some changes to the racial/ethnic composition of the study population over time, but these changes were unlikely to have materially affected our results. For instance, IBC and LABC rates among non-Hispanic and Hispanic whites are similar so the increasing Hispanic percentage of the population should not affect overall results. The percentages of blacks, with higher rates of IBC, and Asian/Pacific Islanders, with lower rates of IBC, also increased slightly but not substantially over time.
Our results suggest that factors influencing the incidence of other types of breast cancer, particularly more advanced disease, may also be operating for IBC. Evidence suggests that some well-established breast cancer risk factors whose prevalence has changed over time are differentially associated with pre- and post-menopausal breast cancer, ER+ and ER− breast cancer, and some possibly with IBC versus other types of breast cancer. Among these are parity, older age at first birth, and increased body mass index.
Giving birth and number of full-term pregnancies have been associated with greater reductions in risk of post-menopausal than pre-menopausal breast cancer [27] and more consistently with reduced risk of ER+ than ER− breast cancer [28]. Few studies have examined associations by ER status among younger women, but those that have found increased parity associated with reduced risk of ER+ breast cancer, but less consistently so with triple-negative breast cancer [29]. Older age at first birth has been associated with greater increases in risk of pre-menopausal than post-menopausal breast cancer [27] as well as ER+ than ER− breast cancer [28]. One study in young women found older age at first birth to be associated with reduced risk of triple negative breast cancer but not ER+ breast cancer [29]. A study of risk factors for IBC found that associations with nulliparity and older age at first livebirth did not vary by menopausal status, but were associated with statistically significant reductions in risk of ER− but not ER+ IBC; these factors were not significantly associated with either ER+ or ER− LABC [30].
U.S. women born in 1935 (aged 66 in 2001) tended to have larger families (with 4 or more children being the most frequent (37%)) than women born in 1960 (aged 41 in 2001), for whom two births per family was the most common (35%) [31]. Hispanic and black women have long been more likely than white women to begin childbearing at an earlier age, with this disparity increasing over time [32]. Among U.S. women, the median age at first birth increased from about 22 years for women born in 1939 (who would be aged 62 in 2001) to 26 years for women born in the late 1960s (approximately 33 years in 2001), after which it declined to about 25 years for birth cohorts through the mid-1970s (approximately 28 years in 2001) and then rose again to about 27 years for birth cohorts through the early 1980s (approximately 21 years in 2001) [32]. The general decline in the number of children and increase in age at first birth over the generations might contribute to the decline in incidence rates of ER− IBC. These changes may also contribute to the increase in risk of ER+ premenopausal breast cancers, but not necessarily to declining trends in older ER+ breast cancer or ER− breast cancer.
Obesity has been associated with increased risk of hormone receptor-positive post-menopausal breast cancer and reduced risk of hormone receptor-positive pre-menopausal breast cancer. No associations in pre- or post-menopausal women have been reported for hormone-receptor negative breast cancer [33]. Obesity has been linked to a more substantial increase in risk of IBC than other types of breast cancer in both pre- and post-menopausal women and those with ER+ and ER− breast cancer [30]. Age-period-cohort analyses of obesity prevalence among U.S. women showed an increase in the prevalence of obesity of 21.6 percent between 1984-2014; this was determined to be a period effect, which was similar across sex and racial/ethnic groups, although somewhat larger among African-Americans [34]. The increasing prevalence of obesity is not consistent with any of the trends we report except the generally increasing rates of other localized breast cancer in older women. Diabetes is a more tentative risk factor for breast cancer, but it has been associated with increased risk of IBC, LABC and other advanced tumors in elderly women [35], although these results were not completely adjusted for BMI. The entire increase in prevalence of diabetes among women in the United States between 1976-1980 and 2007-2010 was accounted for by changes in age, race/ethnicity, and BMI [36].
In conclusion, rates of both ER+ and ER− IBC have declined since the turn of the 21st century, except for ER+ tumors among women younger than age 45. There were no striking differences in trends with other breast cancer types, except for ER+ localized breast cancer in older women. We did not have data to assess why these changes are occurring, but it may be that decreasing parity and rising prevalence of older age at first birth contribute to declining rates of ER− IBC. Otherwise, the patterns of changing risk factors, including the rising prevalence of obesity, which has been associated with increased risk of both ER+ and ER− IBC, do not appear consistent with the trends we observed. These results suggest that further studies of IBC are necessary to identify risk factors and possible preventive strategies.
Supplementary Material
Acknowledgements
Sarah Aurit was supported by the Cancer Epidemiology Education in Special Populations (CEESP) Program; Grant R25 CA112383. This work was also supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health. We thank David Check of the Division of Cancer Epidemiology and Genetics, National Cancer Institute for creating the figure.
Footnotes
The authors declare that this analysis complies with the current laws of the United States.
The authors declare that they have no conflict of interest.
References
- 1.Anderson WF, Schairer C, Chen BE, Hance KW, Levine PH (2005) Epidemiology of inflammatory breast cancer (IBC). Breast Dis 22:9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG et al. , editors (2002) AJCC Cancer Staging Manual. 6th ed. New York, NY: Springer. [Google Scholar]
- 3.Anderson WF, Chu KC, Chang S (2003) Inflammatory breast carcinoma and noninflammatory locally advanced breast carcinoma: distinct clinicopathologic entities? J Clin Oncol 21:2254–2259. [DOI] [PubMed] [Google Scholar]
- 4.Hance KW, Anderson WF, Devesa SS, Young HA, Levine PH (2005) Trends in inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program at the National Cancer Institute. J Natl Cancer Inst 97:966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang S, Parker SL, Pham T, Buzdar AU, Hursting SD (1998) Inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program of the National Cancer Institute, 1975-1992. Cancer 82:2366–2372. [PubMed] [Google Scholar]
- 6.Goldner B, Behrendt CE, Schoellhammer HF, Lee B, Chen SL (2014) Incidence of inflammatory breast cancer in women, 1992-2009, United States. Ann Surg Oncol 21:1267–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hou N, Huo D (2013) A trend analysis of breast cancer incidence rates in the United States from 2000 to 2009 shows a recent increase. Breast Cancer Res Treat 138:633–641. [DOI] [PubMed] [Google Scholar]
- 8.Yeh ED, Jacene HA, Bellon JR, Nakhlis F, Birdwell RL, Georgian-Smith D, Giess CS, Hirshfield-Bartek J, Overmoyer B, Van den Abbeele AD (2013) What radiologists need to know about diagnosis and treatment of inflammatory breast cancer: a multidisciplinary approach. RadioGraphics 33:2003–2017. [DOI] [PubMed] [Google Scholar]
- 9.Beahrs OH, Carr DT, Rubin P, editors (1977) Manual for Staging of Cancer. 1st ed. Chicago, IL: American Joint Committee for Cancer Staging and End Results Reporting. [Google Scholar]
- 10.Beahrs OH, Myers MH, editors (1983). Manual for Staging of Cancer. 2nd ed. Philadelphia, PA: J.B. Lippincott. [Google Scholar]
- 11.Beahrs OH, Henson DE, Hutter RV, Myers MH, editors (1988) Manual for Staging of Cancer. 3rd ed. Philadelphia, PA: J.B. Lippincott. [Google Scholar]
- 12.Beahrs OH, Henson DE, Hutter RV, Kennedy BJ, editors (1992) Manual for Staging of Cancer. 4th ed. Philadelphia, PA: J.B. Lippincott. [Google Scholar]
- 13.Fleming ID, Cooper JS, Henson DE, Hutter RV, Kennedy BJ, Murphy GP et al. , editors (1997) AJCC Cancer Staging Manual. 5th ed. Philadelphia,PA: Lippincott-Raven. [Google Scholar]
- 14.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors (2009) AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer. [Google Scholar]
- 15.Shambaugh EM, Gloeckler Ries L, Young JJL, Kruse MA, Platz CE, Ryan RF et al. (1988) SEER Extent of Disease 1988 Codes and Coding Instructions. Rockville, MD: NIH, National Cancer Institute. [Google Scholar]
- 16.The website of the National Cancer Institute [Internet]. Bethesda: Adjusted AJCC 6th ed. T, N, M, and Stage; c2017 [cited 2016. December 21]. Available from: http://seer.cancer.gov/seerstat/variables/seer/ajcc-stage/6th/. [Google Scholar]
- 17.International Agency for Research on Cancer [Internet]. Geneva: Classification of Diseases for Oncology, 3rd Edition (ICD-O-3); c2017 [cited 2017. January 5]. Available from: http://codes.iarc.fr/. [Google Scholar]
- 18.Taylor SH, Walters R (2010) Potential impact of tumor registry rule changes for recording inflammatory breast cancer. Cancer 116 (11 Suppl):2745–2747. [DOI] [PubMed] [Google Scholar]
- 19.Surveillance, Epidemiology, and End Results Surveillance Research Program [Internet], SEER research data record description. [cited 2018. July 6]. Available from:https://seer.cancer.gov/data-software/documentation/seerstat/nov2012/TextData.FileDescription.pdf
- 20.Chen T, Zhang N, Moran MS, Su P, Haffty BG, Yang Q. Borderline ER-positive primary breast cancer gains no significant survival benefit from endocrine therapy: a systematic review and meta-analysis (2017). Clinical Breast Cancer 18(1):1–8. [DOI] [PubMed] [Google Scholar]
- 21.Collaborative Staging Task Force of the American Joint Committee on Cancer [Internet], Collaborative staging manual and coding instructions, v02.05. [cited 2018. July 6]. Available from: https://cancerstaging.org/cstage/coding/Pages/Version-02.05.aspx
- 22.Anderson WF, Katki HA, Rosenberg PS (2011) Incidence of breast cancer in the United States: Current and future trends. J Natl Cancer Inst 103:1397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howlander N, Chen VW, Ries LAG, Loch MM, Lee R, deSantis C, Lin CC, Ruhl J, Cronin KA (2014) Overview of breast cancer collaborative stage data items – their definitions, quality, usage, and clinical implications: A review of SEER data for 2004-2010. Cancer 120 (23 suppl):3771–3780. [DOI] [PubMed] [Google Scholar]
- 24.Kim HJ, Fay MP, Feuer EJ, Midthune DN (2000) Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 19:335–351 (correction: 2001;20:655). [DOI] [PubMed] [Google Scholar]
- 25.Devesa SS, Donaldson J, Fears T (1995) Graphical representation of trends in rates. Am J Epidemiol 141:300–304. [DOI] [PubMed] [Google Scholar]
- 26.Toriola AT, Colditz GA (2013) Trends in breast cancer incidence and mortality in the United States: implications for prevention. Breast Cancer Res Treat 138:665–673. [DOI] [PubMed] [Google Scholar]
- 27.Clavel-Chapelon F, Gerber M (2002) Reproductive factors and breast cancer risk. Do they differ according to age at diagnosis? Breast Cancer Res Treat 72(2):107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME (2004) Etiology of hormone receptor-defined breast cancer: A systematic review of the literature. Cancer Epideiol Biomarkers Prev 13(10):1558–1568. [PubMed] [Google Scholar]
- 29.Li CI, Beaber EF, Chen Tang M-T, Porter PL, Daling JR, Malone KE (2013) Reproductive factors and risk of estrogen receptor positive, triple negative, and HER2-neu overexpressing breast cancer among women 20-44 years of age. Breast Cancer Res Treat 137:579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schairer C, Li Y, Frawley P, Graubard BI, Wellman RD, Buist DS, Kerlikowske K, Onega TL, Anderson WF, Miglioretti DL (2013) Risk factors for inflammatory breast cancer and other invasive breast cancers. J Natl Cancer Inst 105:1373–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirmeyer SE, Hamilton BE (2011) Childbearing differences among three generations of U.S. women. NCHS Data Brief; 68. [PubMed] [Google Scholar]
- 32.Finer LB, Philbin JM (2014) Trends in ages at key reproductive transitions in the United States, 1951-2010. Women’s Health Issues 24-3;e271–e279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A (2014) Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev 36;114–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.An R, Xiang X (2016) Age-period-cohort analyses of obesity prevalence in US adults. Public Health 141:163–169. [DOI] [PubMed] [Google Scholar]
- 35.Schairer C, Gadalla SM, Pfeiffer RM, Moore SC, Engels EA (2017) Diabetes, abnormal glucose, dyslipidemia, hypertension and risk of inflammatory and other breast cancer. Cancer Epidemiol Biomarkers Prev 26:862–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menke A, Rust KF, Fradkin J, Cheng YJ. Cowie CC (2014) Associations between trends in race/ethnicity, aging, and body mass index with diabetes prevalence in the United States. Ann Intern Med 161:328–335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


