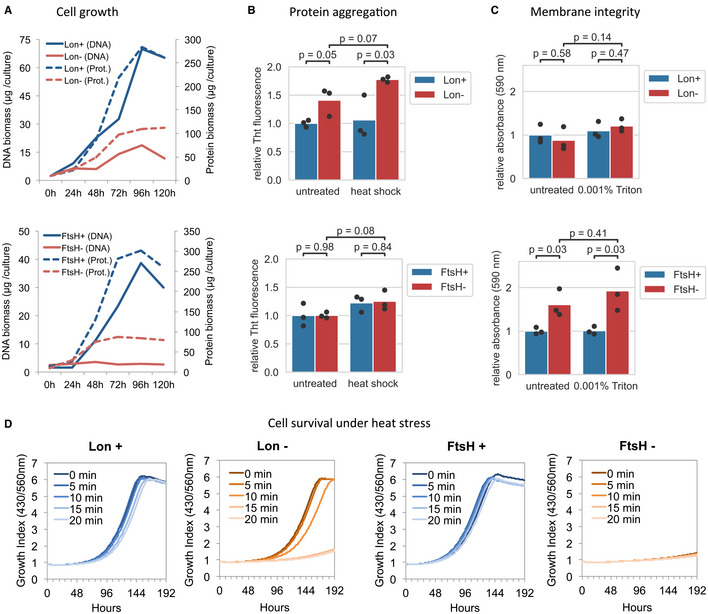
Cell growth assessment of ΔIndLon (upper plot) and ΔIndFtsH (lower plot) mutants grown under inducing (blue) or depleting conditions (red). Growth was monitored by measuring DNA and protein biomass over time. The average from two independent biological replicates is shown.
Protein aggregation in ΔIndLon (upper plot) and ΔIndFtsH (lower plot) mutants grown under inducing (blue) or depleting conditions (red, 48 h, and 72 h of depletion for Lon and FtsH, respectively). Protein aggregates in Triton X‐100 insoluble fractions of untreated or heat‐shock (15 min at 45°C)‐treated cells were measured by Thioflavin (ThT) staining. Bars represent the mean of three biological replicates (dots). Significance of comparisons was assessed by two‐sided independent t‐test (exact P‐values are shown).
Assessment of cell membrane integrity of ΔIndLon (upper plot) and ΔIndFtsH (lower plot) mutants grown under inducing (blue) or depleting conditions (red, 72 h of depletion for both, Lon and FtsH). Membrane integrity of untreated cells or after exposure during 30 min to 0.001% Triton X‐100 was assessed by trypan blue exclusion staining. Bars represent the mean of three biological replicates (dots). Significance of comparisons was assessed by two‐sided independent t‐test (exact P‐values are shown).
Role of Lon and FtsH under heat‐shock stress conditions. ΔIndLon and ΔIndFtsH mutants were grown under inducing (Lon+ or FtsH+) or depleting conditions (Lon‐ or FtsH‐, 60 h of depletion for both, Lon and FtsH), and then exposed at 45°C during 0, 5, 10, 15, or 20 min. Then, growth after the heat treatment was monitored over time under inducing conditions by the 430/560 absorbance rate index that shows pH changes in the medium. The average from two independent biological replicates is shown for each condition.