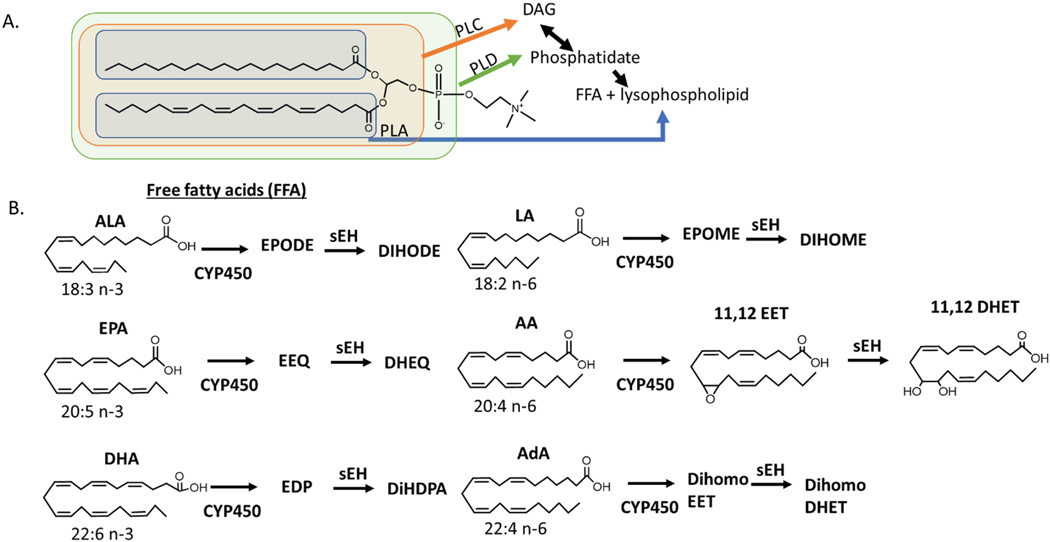
Fig. 5.2.
Formation of EpFA
Polyunsaturated fatty acids (PUFAs) differ in both structure and function based on number of carbons and location of double bonds. They are incorporated as glycerides in fat cells, cellular membranes or circulating micelles and are liberated to free fatty acids by different phospholipases (PL) that act upon different areas of the glyceride or phospholipid (A). EpFA are formed through the oxygenation of FFA by CYP450. The metabolism of Arachidonic Acid (AA, 20:4 n-6) by cytochrome P450 yields EpFAs, epoxyeicosatrienoic acids (EET), which are further degraded by the soluble epoxide hydrolase into dihydroxyeicosatrienoic acids (DHET). The epoxide and diol on the 11,12 position is shown, but similar regioisomers are possible on all the double bonds in the PUFA. The n-6 fatty acid linoleic acid, LA, has been attributed to largely inflammatory epoxides, EPOMES; however, recent studies show that they are only toxic in the presence of sEH, suggesting that the diols of LA, DIHOMES, are responsible for this inflammatory action [137]. The 18:3 omega-3 fatty acid, linolenic acid (ALA), does not seem to have this same inflammatory action. The omega-6 fatty Adrenic Acid, AdA, is named for its abundance in the adrenal gland. Less is known about this PUFA and its metabolites, although the AdA EpFA, dihomoEETs are thought to regulate blood flow to the adrenal gland [138]. n-3 fatty acids alpha eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) form EpFA epoxyeicosatetraenoic acids (EEQs) and epoxydocosapentaenoic acids (EDPs) respectively. Omega-3 EpFA are largely beneficial with anti-inflammatory and pain resolving properties in vitro and in vivo. Little biological activity has been associated with the diols of Ada (dihomoDHET) or the diols of EPA and DHA, dihydroxyeicosatetraenoic acids (DHEQs) and dihydroxydocosapentaenoic acids (DiHDPAs) respectively. (see [86] for review)