Table 5.2.
Clinical development candidates of small molecule sEHI and EpFA mimics
| Small molecule sEH inhibitors | Clinical Trials | |
|---|---|---|
| AUDA | 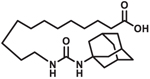 |
Clinical trial
NCT00654966: Microvessel Tone in patients with heart failure. Status: Complete. Healthy humans and patients with heart failure challenged with topical urotensin II, a potent vasoconstrictor, and treated with sEHI [74]. |
| TPPU | This compound has not been investigated in clinical trials but is the most frequently used compound in preclinical research. | |
| AR9281 | 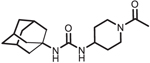 |
Clinical trial
NCT00847899: Evaluation of sEHI in Patients with Hypertension and Impaired Glucose Tolerance. Status: Complete. Single and multiple day testing up to 8 days at doses up to 1.2 g/day (400 mg every 8 h) were well tolerated [129]. Data from Phase II clinical trials not published. |
| GSK2256294 | 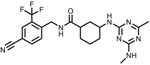 |
Clinical trial
NCT01762774: A Study to Assess the Safety,
Tolerability, PK and PD of Single and Repeat Doses of GSK2256294 in Healthy Volunteers and Adulate Male Moderately Obese Smokers. Status: Complete. Doses were well tolerated and attenuated smoking related endothelial dysfunction. |
| EC1728 (t-TUCB) | Clinical trials are in development for companion animals. | |
| EC5026 | 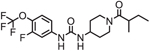 |
Clinical trial
NCT04228302: Safety, Tolerability, and Pharmacokinetics of Oral EC5026 in Healthy Subjects. Status: Enrolling |
| EpFA Mimics | Summary of results | |
| CMX020 | 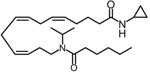 |
Clinical trial ACTRN12615000885594: A Study to Evaluate the Safety and Analgesic Efficacy of Oral CMX-020 in Subjects with Symptoms of Sciatica Resulting from Lumbosacral Radiculopathy. ACTRN12616001435471: A Phase 2 Study to Assess the Efficacy and Safety of CMX-020 in Treating Osteoarthritis. Status: Phase 1 studies complete. Enrolling for Phase 2. |
| OMT-28 | Structure not disclosed |
Clinical trial
NCT03906799: Study on OMT-28 in Maintenance of Sinus Rhythm in Patients with Persistent Atrial Fibrillation. Status: Enrolling |
| Icosabutate |
Clinical trial
NCT04052516: A Phase 2b Study of Icosabutate in Fatty Liver Disease. Status: Phase 1 studies complete. Enrolling for Phase 2b. |
|
| Vascepa (ethyl-EPA) | Status: approved for the treatment of hypertriglyceridemia. | |
