Abstract
X inactive specific transcript (XIST) is a novel long noncoding RNA (lncRNA) which has been reported to be frequently upregulated in various human cancer types and to function as an oncogene. It has been reported that the expression of lncRNA XIST was upregulated in non-small cell lung cancer (NSCLC). In the present study, we aimed to investigate the clinical significance and prognostic value of XIST in patients with NSCLC.
A total of 156 pairs of NSCLC and corresponding adjacent normal lung tissue samples were obtained from NSCLC patients who had undergone surgery from July 2014 to March 2019. The Student's t test was used in different treated groups for statistical analysis. The association between XIST expression and clinicopathological features of NSCLC patients was evaluated using the chi-squared test. Survival curves were plotted using Kaplan-Meier method and compared by log-rank test.
The expression of XIST was significantly higher in NSCLC samples compared to non-cancerous samples (P < .001). Statistically significant correlations were observed between high tissue XIST expression level and lymph node metastasis (P = .036) and high Tumor Node Metastasis (TNM) stage (P = .002). The log-rank test indicated that patients with increased XIST expression experienced poor overall survival (P = .006). Multivariate Cox regression analysis showed that XIST expression level (hazard ratio = 2.645, 95% confidence interval: 1.672–7.393, P = .029) was an independent factors in predicting the overall survival of NSCLC patients.
The present study found that XIST expression level was significantly associated with advanced pathological stage and high TNM stage in NSCLC. Furthermore, upregulation of tissue lncRNA XIST predicts poor postoperative survival in patients with NSCLC.
Keywords: clinical significance, long noncoding RNAs, lung cancer, non-small cell lung cancer, prognosis, X inactive specific transcript
1. Introduction
Lung cancer ranks the most common cancer all over the world and its most common type is non-small cell lung cancer (NSCLC).[1] According to WHO classification, lung adenocarcinoma (LUAD) and lung squamous cell cancer (LSCC) as two major histological types of NSCLC 4, and LUAD has become the most frequent histological type of NSCLC.[2] Although great progress in diagnostic and therapeutic methods has been made, the prognosis of NSCLC patients after surgery remains dismal.[3–5] Therefore, it is essential to explore new targets for NSCLC diagnosis and treatment.
Long noncoding RNAs (lncRNAs) stand for a class of transcribed RNA molecules with length of more than 200 nucleotides.[6] LncRNAs encode no protein products but regulate gene expression at multiple levels. LncRNAs are found to be involved in a diverse aspect of the biology, such as gene expression regulation, chromatin structure, epigenetic control, and splicing.[7,8] In addition, aberrant levels of lncRNAs can also play a key role in the pathological progression of many diseases, including a variety of human cancers.[9,10]
In recent years, the lncRNA X inactive specific transcript (XIST) has been reported to be frequently upregulated in certain common types of cancer, and its upregulation is associated with tumour progression and poor prognosis of cancer patients, including cervical cancer, bladder cancer, pancreatic cancer, colorectal cancer, ovarian cancer, gastric cancer, renal cell carcinoma, hepatocellular carcinoma, and oesophageal squamous cell carcinoma.[11–19]
The expression level XIST has been found to be up-regulated in NSCLC, and downregulation of XIST inhibited cell proliferation, migration, invasion and EMT of NSCLC.[20–24] In the present study, we aimed to investigate the clinical significance and prognostic value of XIST in patients with NSCLC.
2. Materials and methods
2.1. Patients and tissue specimens
A total of 156 pairs of NSCLC and corresponding adjacent normal lung tissue samples were obtained from 156 NSCLC patients who had undergone surgery at the Department of Cardiothoracic surgery, Ruikang Hospital Affiliated to Guangxi University of Chinese Medicine from July 2014 to March 2019. None of these patients had received chemotherapy or radiotherapy before surgery. The collected tissue samples were confirmed by histopathological examination, immediately snap frozen in liquid nitrogen and stored at −80°C until required. This study was approved by the Ethical Committee of Ruikang Hospital Affiliated to Guangxi University of Chinese Medicine. Clinical data was obtained from medical record of patients. The clinicopathological characteristics of these patients were listed in Table 1.
Table 1.
Relationship between the levels of XIST and clinicopathological variables in NSCLC patients.
| XIST expression | ||||
| Variables | Cases(n) | High(n = 79) | Low(n = 77) | P value |
| Gender | ||||
| Male | 83 | 43 | 40 | .873 |
| Female | 73 | 36 | 37 | |
| Age (yr) | ||||
| < 60 | 68 | 28 | 40 | .052 |
| ≥60 | 88 | 51 | 37 | |
| Smoking history | ||||
| Positive | 65 | 35 | 30 | .52 |
| Negative | 91 | 44 | 47 | |
| Family history of lung cancer | ||||
| No | 75 | 34 | 41 | .262 |
| Yes | 81 | 45 | 36 | |
| Tumor size (cm) | ||||
| <3 | 68 | 30 | 38 | .196 |
| ≥3 | 88 | 49 | 39 | |
| Lymph Node Metastasis | ||||
| Negative | 69 | 28 | 41 | .036 |
| Positive | 87 | 51 | 36 | |
| TNM stage | ||||
| I/II | 71 | 26 | 45 | .002 |
| III | 85 | 53 | 32 | |
2.2. Quantitative real-time RT-PCR analysis (qPCR)
Total RNA was extracted using TRIzol (Invitrogen, USA). cDNA was synthesized using the PrimeScript RT reagent Kit (TaKaRa), and qPCR was performed using the Real-Time Quantitative PCR SYBR Green kit (TaKaRa) on the ABI 7500 fast real-time PCR system (Applied Biosystems, Foster City, CA, USA). PCR reactionconditions were: 55 seconds at 95°C, and then 12 seconds at 95°C and 32 seconds at 58.5°C for 40 cycles. The expression of XIST was normalized to GAPDH using 2-ΔΔCT method. Sequences of primers used in PCR reactions were: XIST, forward: 5′-ACGCTGCATGTGTCCTTAG-3, reverse: 5′-GAGCCTCTTATAGCTGTTTG-3′; GAPDH, forward: 5′-GGAATCCACTGGCGTCTTCA-3′, reverse: 5′-GGTTCACGCCCATCACAAAC-3′.
2.3. Statistical analysis
Statistical analysis was performed using GraphPad Prism 6.0 software (GraphPad Software, San Diego, CA) and SPSS statistical software, version 19.0 (SPSS Inc., Chicago, IL). The Student's t test was used in different treated groups for statistical analysis. The association between XIST expression and clinicopathological features of NSCLC patients was evaluated using the chi-squared test. Survival curves were plotted using Kaplan-Meier method and compared by log-rank test. P < .05 was considered to indicate a statistically significant difference.
3. Results
3.1. The expression level of XIST in NSCLC tissues and adjacent normal tissues
We assessed XIST expression in 156 NSCLC samples and 156 paired non-cancerous samples. The expression of XIST was significantly higher in NSCLC samples compared to non-cancerous samples (P < .0001, shown in Fig. 1). We divided 156 NSCLC patients into two groups according to the levels of XIST expression level. The cut-off point was the median expression level of XIST in NSCLC samples (high expression group, n = 79; low expression group, n = 77).
Figure 1.
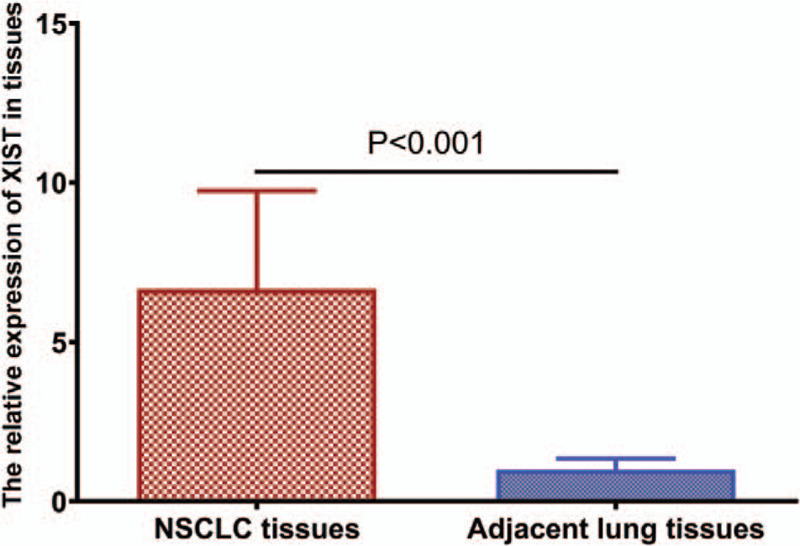
Relative levels of XIST in NSCLC tissues and adjacent non-tumor tissues were determined by quantitative RT-PCR (qRT-PCR).
3.2. The correlation between XIST expression level and clinicopathological features of NSCLC patients
We then investigated the associations of tissue XIST expression level with various clinicopathological characteristics of human NSCLC. Statistically significant correlations were observed between high tissue XIST expression level and lymph node metastasis (P = .036) and high TNM stage (P = .002; shown in Table 1). However, there was no significant association between XIST expression level and other clinicopathological parameters, including age, gender, tumor size, smoking history, and family history of lung cancer (all P > .05; shown in Table 1).
3.3. Increased XIST expression level predicts the poor prognosis of NSCLC patients
Using kaplan-Meier survival plots and log-rank analyses, we evaluated the association of XIST expression with overall survival. The log-rank test indicated that patients with increased XIST expression experienced poor overall survival (P = .006, shown in Figure 2). To determine the possibility of XIST as an independent risk factor for poor prognosis, both clinicopathological factors and the level of XIST expression were evaluated by multivariate Cox regression analysis. Results showed that XIST expression level (HR = 2.645, 95%CI: 1.672–7.393, P = .029) was an independent factors in predicting the overall survival of NSCLC patients (shown in Table 2).
Figure 2.
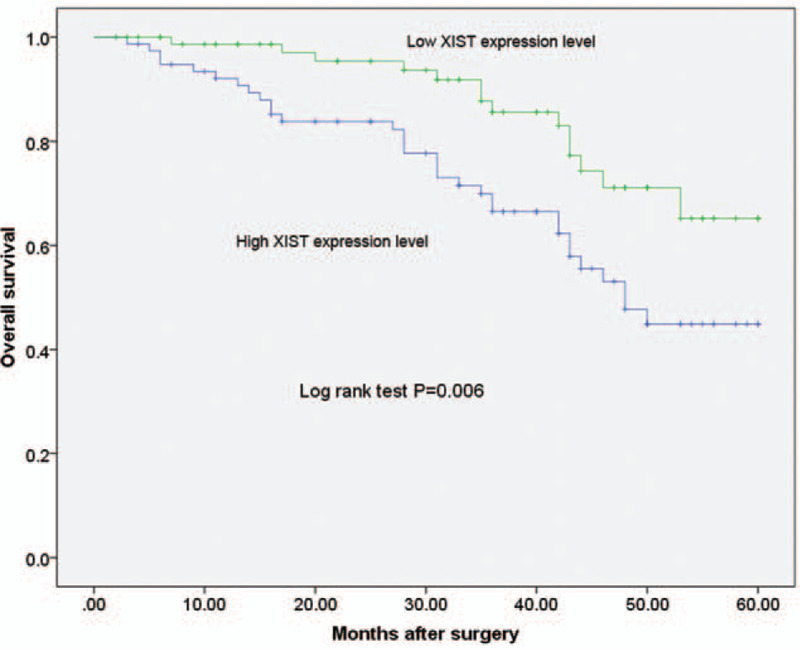
Prognostic value of XIST for NSCLC patients was analyzed by Kaplan-Meier analysis.
Table 2.
Multivariate analysis of overall survival in patients with NSCLC.
| Variable | Hazard ratio | 95% CI | P value |
| Gender | 0.784 | 0.563–1.357 | .583 |
| Age | 1.749 | 0.722–3.855 | .129 |
| Smoking history | 1.937 | 0.751–3.559 | .564 |
| Family history of lung cancer | 2.744 | 0.821–5.682 | .143 |
| Tumor size | 1.693 | 0.832–4.229 | .088 |
| Lymph Node Metastasis | 3.672 | 2.832–11.943 | .009 |
| TNM stage | 3.829 | 2.553–14.893 | .005 |
| XIST expression level | 2.645 | 1.672–7.393 | .029 |
4. Discussion
NSCLC is one of the most common malignancies around the globe. The pathogenesis of NSCLC is a multistep and multistage process involving environmental factors and genetic factors. Despite numerous years of basic and clinical research on NSCLC, the 5-year survival rate still remains less than 15%.[25,26] Therefore, exploring the molecular mechanisms and searching for key molecules during initiation and progression of NSCLC are important for the development of novel treatment.
Long non-coding RNAs, RNA transcripts longer than 200 bp in length, are the major part of transcribed noncoding RNA.[27] Previous studies demonstrated that lncRNAs are essential players in the pathogenesis of human diseases, particularly different types of human cancer.[28,29] Therefore, lncRNAs may be promising therapeutic targets for the treatment of cancer.
XIST is an lncRNA required for transcriptional silencing of the X chromosome, which plays an important role in the inactivation of the X chromosome. In recent years, increasing evidence has shown that XIST is closely related to the occurrence and progression of many human cancers. For example, Zhang et al found that lncRNA XIST was found to act as a miR-497–5p sponge and to regulate the level of PDCD4, which is targeted by miR-497-5p. lncRNA XIST was observed to be downregulated in the HCC tissues and positively correlated with the expression of PDCD4, revealing that the XIST/miR-497-5p/PDCD4 axis participates in HCC development and that XIST could be used as a biomarker of HCC.[18] Chen et al found that the effects of XIST/miR-140-5p/ORC1 axis on the progression of cervical cancer which will shed new light on epigenetic diagnostics and therapeutics in cervical cancer.[11] Sun et al found that XIST was down-regulated in RCC tissues and cells. Overexpression of XIST significantly suppressed cell proliferation and induced cell G0/G1 arrest in vitro and inhibited tumor growth in vivo. They further found that XIST could directly interact with miR-106b-5p and increase the expression of P21. Thus, XIST positively regulated the expression of P21 through sponging miR-106b-5p, and played a tumor suppressor role in RCC. Moreover, they found that curcumin could regulate XIST/miR-106b-5p/P21 axis in RCC cells.[17] Chen et al found that lncRNA XIST was significantly up-regulated in gastric cancer tissues and cell lines. Overexpression of lncRNA XIST was markedly associated with larger tumor size, lymph node invasion, distant metastasis and TNM stage in gastric cancer patients. Functionally, knockdown of lncRNA XIST exerted tumor-suppressive effects by inhibiting cell proliferation, migration and invasion in vitro and tumor growth and metastasis in vivo. Furthermore, an inverse relationship between lncRNA XIST and miR-101 was found. Polycomb group protein enhancer of zeste homolog 2 (EZH2), a direct target of miR-101, could mediated the biological effects that lncRNA XIST exerted.[30] Zuo et al found that XIST was up-regulated in epithelial ovarian cancer (EOC) tissues and cell lines. The expression of XIST was closely related to the tumor grade, distant metastasis, and FIGO stage in the EOC patients. The Cox regression analysis showed that high XIST expression was an independent predictor of prognosis in patients with EOC. In in vitro experiments, reducing XIST expression significantly suppressed cell proliferation, migration and invasion in EOC cells.[19] Shen et al found that XIST was frequently upregulated while miR-429 was commonly downregulated in pancreatic cancer tissues, especially in metastatic pancreatic cancer tissues. Knockdown of XIST in two pancreatic cancer cell lines caused inhibition of migration, invasion and EMT capacities. Forced expression of miR-429 exerted the similar tumor suppressing effects. XIST repressed miR-429 expression thus upregulated ZEB1, one of the targets of miR-429. ZEB1 mediated the tumor suppressing roles of XIST knockdown in pancreatic cancer cells. They identified the critical axis of XIST/miR-429/ZEB1 in pancreatic cancer cell migration, invasion and EMT, which may aid in developing new therapeutic strategies for pancreatic cancer.[16]
The expression level XIST has been found to be up-regulated in NSCLC, and downregulation of XIST inhibited cell proliferation, migration, invasion and EMT of NSCLC. Furthermore, upregulation of lncRNA-XIST was associated with cisplatin resistance in NSCLC by downregulating miRNA-144-3p in H460/DDP and A549/DDP cells, a murine A549/DDP tumor xenograft, and human tumor tissues from patients with cisplatin-resistant NSCLC.[20] In the present study, we aimed to investigate the clinical significance and prognostic value of XIST in patients with NSCLC. We assessed XIST expression in 156 NSCLC samples and 156 paired non-cancerous samples. The expression of XIST was significantly higher in NSCLC samples compared to non-cancerous samples. We then investigated the associations of tissue XIST expression level with various clinicopathological characteristics of human NSCLC. Statistically significant correlations were observed between high tissue XIST expression level and lymph node metastasis and high TNM stage. However, there was no significant association between XIST expression level and other clinicopathological parameters, including age, gender, tumor size, smoking history, and family history of lung cancer. Using kaplan-Meier survival plots and log-rank analyses, we evaluated the association of XIST expression with overall survival. The log-rank test indicated that patients with increased XIST expression experienced poor overall survival. To determine the possibility of XIST as an independent risk factor for poor prognosis, both clinicopathological factors and the level of XIST expression were evaluated by multivariate Cox regression analysis. Results showed that XIST expression level was an independent factors in predicting the overall survival of NSCLC patients.
In conclusions, the present study found that XIST expression level was significantly associated with advanced pathological stage and high TNM stage in NSCLC. Furthermore, upregulation of tissue lncRNA XIST predicts poor postoperative survival in patients with NSCLC.
Author contributions
Data curation: Hengxiao Fang.
Formal analysis: Liushan Yang, Yue Fan.
Investigation: Liushan Yang, Yue Fan.
Methodology: Hengxiao Fang.
Resources: Chunrong Mo, Lei Luo.
Software: Hengxiao Fang, Daying Liang.
Writing – original draft: Daying Liang, Hengxiao Fang.
Writing – review & editing: Yi Jiang.
Footnotes
Abbreviations: 95%CI = 95% confidence interval, HR = hazard ratio, lncRNAs = long noncoding RNAs, NSCLC = non-small cell lung cancer, OSCC = ovarian squamous cell carcinoma, qPCR = Quantitative real-time RT-PCR analysis, SCLC = small cell lung cancer, XIST = X inactive specific transcript.
How to cite this article: Fang H, Yang L, Fan Y, Mo C, Luo L, Liang D, Jiang Y. Upregulation of tissue long noncoding RNA X inactive specific transcript predicts poor postoperative survival in patients with non-small cell lung cancer. Medicine. 2020;99:50(e21789).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol 2005;40:90–7. [DOI] [PubMed] [Google Scholar]
- [3].Carrizosa DR, Gold KA. New strategies in immunotherapy for non-small cell lung cancer. Transl Lung Cancer Res 2015;4:553–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet 2017;389:299–311. [DOI] [PubMed] [Google Scholar]
- [5].Minguet J, Smith KH, Bramlage P. Targeted therapies for treatment of non-small cell lung cancer--Recent advances and future perspectives. Int J Cancer 2016;138:2549–61. [DOI] [PubMed] [Google Scholar]
- [6].Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell 2018;172:393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Beermann J, Piccoli MT, Viereck J, et al. Non-coding RNAs in development and disease: background, mechanisms, and therapeutic approaches. Physiol Rev 2016;96:1297–325. [DOI] [PubMed] [Google Scholar]
- [8].Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet 2014;15:7–21. [DOI] [PubMed] [Google Scholar]
- [9].Mirhosseini SA, Sarfi M, Samavarchi Tehrani S, et al. Modulation of cancer cell signaling by long noncoding RNAs. J Cell Biochem 2019;120:12224–46. [DOI] [PubMed] [Google Scholar]
- [10].Yang G, Lu X, Yuan L. LncRNA: a link between RNA and cancer. Biochim Biophys Acta 2014;1839:1097–109. [DOI] [PubMed] [Google Scholar]
- [11].Chen X, Xiong D, Ye L, et al. Up-regulated lncRNA XIST contributes to progression of cervical cancer via regulating miR-140-5p and ORC1. Cancer Cell Int 2019;19:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hu B, Shi G, Li Q, et al. Long noncoding RNA XIST participates in bladder cancer by downregulating p53 via binding to TET1. J Cell Biochem 2019;120:6330–8. [DOI] [PubMed] [Google Scholar]
- [13].Li J, He D. Long noncoding RNA Xist predicts the presence of lymph node metastases in human oesophageal squamous cell carcinoma. Br J Biomed Sci 2019;76:147–9. [DOI] [PubMed] [Google Scholar]
- [14].Li Y, Zhang Q, Tang X. Long non-coding RNA XIST contributes into drug resistance of gastric cancer cell. Minerva Med 2019;110:270–2. [DOI] [PubMed] [Google Scholar]
- [15].Liu A, Liu L, Lu H. LncRNA XIST facilitates proliferation and epithelial-mesenchymal transition of colorectal cancer cells through targeting miR-486-5p and promoting neuropilin-2. J Cell Physiol 2019;234:13747–61. [DOI] [PubMed] [Google Scholar]
- [16].Shen J, Hong L, Yu D, et al. LncRNA XIST promotes pancreatic cancer migration, invasion and EMT by sponging miR-429 to modulate ZEB1 expression. Int J Biochem Cell Biol 2019;113:17–26. [DOI] [PubMed] [Google Scholar]
- [17].Sun K, Jia Z, Duan R, et al. Long non-coding RNA XIST regulates miR-106b-5p/P21 axis to suppress tumor progression in renal cell carcinoma. Biochem Biophys Res Commun 2019;510:416–20. [DOI] [PubMed] [Google Scholar]
- [18].Zhang Y, Zhu Z, Huang S, et al. lncRNA XIST regulates proliferation and migration of hepatocellular carcinoma cells by acting as miR-497-5p molecular sponge and targeting PDCD4. Cancer Cell Int 2019;19:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zuo K, Zhao Y, Zheng Y, et al. Long non-coding RNA XIST promotes malignant behavior of epithelial ovarian cancer. Onco Targets Ther 2019;12:7261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tian LJ, Wu YP, Wang D, et al. Upregulation of long noncoding RNA (lncRNA) X-inactive specific transcript (XIST) is associated with cisplatin resistance in non-small cell lung cancer (NSCLC) by downregulating MicroRNA-144-3p. Med Sci Monit 2019;25:8095–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Qiu HB, Yang K, Yu HY, et al. Downregulation of long non-coding RNA XIST inhibits cell proliferation, migration, invasion and EMT by regulating miR-212-3p/CBLL1 axis in non-small cell lung cancer cells. Eur Rev Med Pharmacol Sci 2019;23:8391–402. [DOI] [PubMed] [Google Scholar]
- [22].Zhou X, Xu X, Gao C, et al. XIST promote the proliferation and migration of non-small cell lung cancer cells via sponging miR-16 and regulating CDK8 expression. Am J Transl Res 2019;11:6196–206. [PMC free article] [PubMed] [Google Scholar]
- [23].Liu J, Yao L, Zhang M, et al. Downregulation of LncRNA-XIST inhibited development of non-small cell lung cancer by activating miR-335/SOD2/ROS signal pathway mediated pyroptotic cell death. Aging (Albany NY) 2019;11:7830–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang J, Cai H, Dai Z, et al. Down-regulation of lncRNA XIST inhibits cell proliferation via regulating miR-744/RING1 axis in non-small cell lung cancer. Clin Sci (Lond) 2019;133:1567–79. [DOI] [PubMed] [Google Scholar]
- [25].Park C, Lee IJ, Jang SH, et al. Factors affecting tumor recurrence after curative surgery for NSCLC: impacts of lymphovascular invasion on early tumor recurrence. J Thorac Dis 2014;6:1420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sherwood J, Dearden S, Ratcliffe M, et al. Mutation status concordance between primary lesions and metastatic sites of advanced non-small-cell lung cancer and the impact of mutation testing methodologies: a literature review. J Exp Clin Cancer Res 2015;34:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet 2009;10:155–9. [DOI] [PubMed] [Google Scholar]
- [28].Qi P, Zhou XY, Du X. Circulating long non-coding RNAs in cancer: current status and future perspectives. Mol Cancer 2016;15:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Spizzo R, Almeida MI, Colombatti A, et al. Long non-coding RNAs and cancer: a new frontier of translational research? Oncogene 2012;31:4577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chen DL, Ju HQ, Lu YX, et al. Long non-coding RNA XIST regulates gastric cancer progression by acting as a molecular sponge of miR-101 to modulate EZH2 expression. J Exp Clin Cancer Res 2016;35:142. [DOI] [PMC free article] [PubMed] [Google Scholar]


