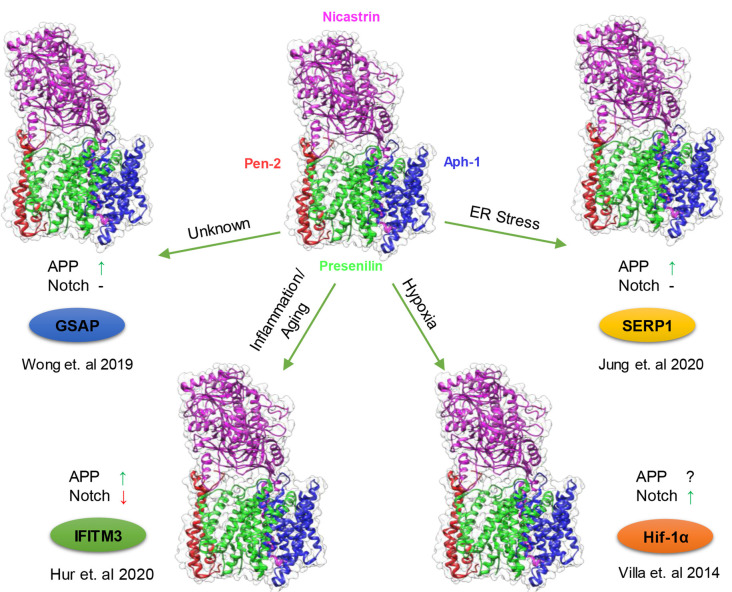
Figure 2.
Modulation of γ-secretase by γ-secretase modulatory proteins (GSMPs). Schematic representation of the conditions in which GSMP triggers the attenuation of γ-secretase activity and selectivity. Cells possess a different type of γ-secretase complexes with variable levels of activities (PDB structure: 6IDF), which under certain cellular circumstances GSMP can bind and modify its activity and selectivity (clockwise): endoplasmic reticulum (ER) stress upregulate stress-associated ER protein 1 (SERP1) expression which binds and localize the γ-secretase complex to lipid rafts where amyloid precursor protein (APP) resides thus increasing APP cleavage but not Notch processing (Jung et al., 2020), hypoxic conditions stabilize Hif-1α which in turn binds to γ-secretase and increase its activity for Notch substrates (Villa et al., 2014), innate immune response and aging triggers the binding of interferon-induced transmembrane protein 3 (IFITM3) thus enhancing γ-secretase for APP but reducing Notch processing (Hur et al., 2020) and under unknown conditions GSAP attenuates γ-secretase activity solely towards APP but not Notch (Wong et al., 2019; Magenta—Nicastrin, Green—Presenilin, Blue—Aph-1 and Red—Pen-2).