Abstract
Artificial bone grafts possess the advantages of good biodegradability, customizable dimensions, and sufficient mechanical properties, which can promote cell proliferation and differentiation in bone tissue regeneration. 3D printing is a delicate approach that endows the scaffolds with excellent controllability and repeatability when compared with conventional bio-fabrication methods. However, the limitation of printing resolution somehow makes it difficult to prepare bone defect substitution with high porosity and hierarchical construct. In this study, we utilized polylactic acid (PLA) as printing materials and developed a smart strategy to combine 3D printing technology with bio-fabrication methods. A porous planar scaffold was printed and then rolled up into a spiral structure with adjustable pore size and porosity. The topographic features and morphology of the artificial scaffolds were examined through stereomicroscope and SEM, respectively. The porous spiral scaffold presented good mechanical properties in a set of mechanical testing. Later, the human fetal osteoblasts (hFOB) were cultured on the porous spiral scaffold and its control groups for a total of 28 days. The MTS analysis, alkaline phosphatase (ALP) assay, and alizarin red S (ARS) staining were used to analyze the cell proliferation, osteogenic differentiation, and mineral deposition after a certain period of time. The results indicated that compared with the other two scaffolds, the porous spiral scaffold with larger surface area and better interconnections between internal porous networks could significantly improve the spatial cell compartment and promote cell growth and differentiation. The porous spiral scaffold may see versatile applications in large-volume bone defects regeneration.
Keywords: Polylactic acid, scaffold, porous, bone regeneration, 3D printing
Graphical Abstract
1. Introduction
Bone tissue engineering offers great potential in the development of bone substitutes which have the ability to provide better mechanical strength, biocompatibility, and osteoinductivity. 1, 2 Due to some serious conditions, such as trauma, infection, and surgical resections, large-scale tissue-engineered bone grafts for rapid osteogenesis are in high demand. Nowadays, compared with autografts and allografts, artificial bone grafts are preferred in bone-grafting operations owing to the low risks of causing donor site scarcity, immune rejection, and pathogen transfer. 3, 4 In cognition of the crucial roles of both extracellular matrix (ECM) and cellular organization in determining native tissue biological and mechanical functions, various nano/microfabrication technologies have been explored to recapitulate such ECM complexities in scaffold design. Scaffolds, as the structural niches to mimic the 3D resident environment of cells, should also possess the ability to provide the cells with appropriate topological and physicochemical cues to form the tissues with high similarity to their natural counterparts. 5, 6
A variety of conventional scaffold fabrication methods, which include salt leaching, gas foaming, lyophilization, solvent casting, and phase separation, have been widely developed to build up 3D scaffolds that are utilized in bone tissue engineering. 7–11 However, the unpredictable properties of scaffolds made by conventional bio-fabrication technologies highly limited their applications. For instance, the high degree of randomness during the processing of scaffolds leads to the lack of proper control over roughness, pore size, suitable mechanical properties, and may be various from batch to batch. 12 In order to overcome those challenges, the extrusion-based 3D printing technique has emerged as an ideal approach that enables accurate control of such properties and can be customized into implantable materials with precise geometry as the bone defect. 13, 14 Numerous biodegradable synthesized and natural materials such as polycaprolactone (PCL), polylactic acid (PLA), polyglycolide (PGA), chitosan and their copolymers have been developed as candidates to construct 3D printed scaffolds. 15–17 In particular, PLA is an FDA approved (generally recognized as safe), eco-friendly, recyclable, and compostable polymer with good mechanical properties. All the significant advantages make PLA an attractive polymer for bone reconstruction applications. 18, 19 The degradation products of PLA have proved to possess high biocompatibility and low cytotoxicity in local tissue during the period of bone tissue regeneration. 20 The great thermal processability, excellent tensile strength, high Young’s modulus, and good cytocompatibility enable PLA polymer as great printing filaments to generate engineered 3D scaffolds with complex hierarchical constructs. 21–23
Although the extrusion-based 3D printing technique attracts much attention for bone tissue engineering, a few potential drawbacks are still related to current approaches. It has been reported that porosity and pore size are the key points to encourage bone ingrowth by offering sufficient nutrients and waste exchanges. 24 Micro-scaled pores (100–400μm) promote the migration of osteoblasts and osteoprogenitors into the scaffold and accelerate the osteoid formation and mineralization. 25 However, 3D-printed scaffolds with high porosity mostly exhibit poorer mechanical properties, which are greatly affected by their internal structural alignment manners. 26 The current 3D-printed scaffold, especially for large-scale bone defects reconstruction, requires high mechanical stability which is made up of tightly compacted printing filaments. Furthermore, the lack of effective channels for materials exchange and new tissue formation led to rapid nutrition depletion and hypoxia. The ‘sweet point’ that can balance the mechanical properties and biological functions needs to be achieved through a subtler way. Furthermore, a highly spiral structure for lamellar bone substitution calls for a more accurate apparatus and more restrictive printing conditions. However, the resolution of the printed polymer constructs is usually conceded to allow for 3D structures with a greater footprint. The minimum printed feature resolution of extrusion-based 3D printing is generally over 100 μm due to the high-pressure resistance in the printing nozzle. 27, 28 The unique organization and orientation of trabecular bone and compact bone give rise to their functionalities. Such structures and arrangements are practically hard to be simulated if we solely rely on the additive manufacturing extrusion-based 3D printing. In order to effectively regenerate lamellar bone-like structures with proper functions, some attempts were developed by combining the salt leaching/lyophilization with extrusion-based 3D printing. 29–31 Although the micro-/nano sized pores were generated on the surface of scaffolds, the unrepeatability and uncontrollable spatial distribution of the pores narrowed their applications.
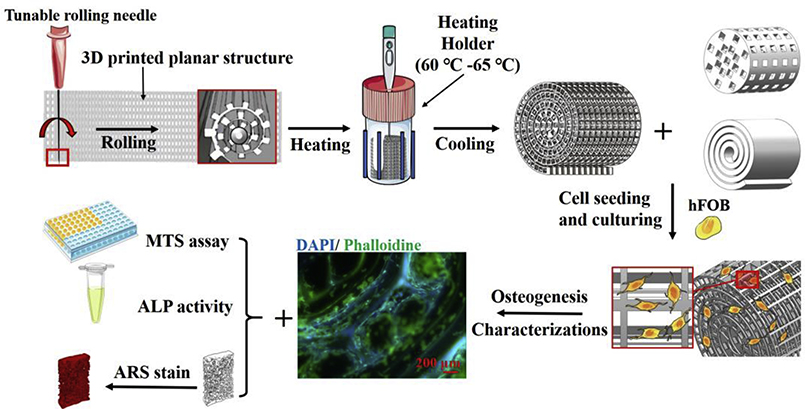
Herein, we developed a straightforward strategy that rolled up the pre-heated 3D printed construct into a porous spiral scaffold to induce the osteogenesis and mineralization for human fetal osteoblast (hFOB) cells. This combination of conventional bio-fabrication technology with extrusion-based 3D printing aimed to produce a porous scaffold that allowed precise control of the spatial organization of various pore size and porosity to achieve multi-functionality and arrangement in the same construct. The arbitrary design on the 2D surface enables the low barrier of printing conditions, while the tunable rolling needle size makes it possible to achieve the adjustable inner core space and different overlays of filaments to form spiral designs. 32, 33 Compared to the porous spiral scaffold, porous cylinder and solid spiral scaffolds were directly printed through 3D printing and were used as the control groups. The characteristics and mechanical strength of three PLA scaffolds were studied. Then, the effects of three PLA scaffolds on osteogenesis and efficiency in forming large-scaled bone tissue were further investigated. With the further demonstration of such osteoinductivity and osteoconductivity, we expect that the porous spiral scaffold may see versatile applications in large-volume bone defects regeneration.
2. Experimental Section
2.1. Materials.
Polylactic acid (PLA) filament (1.75 mm diameter) was obtained from Thermo Fisher Scientific (Waltham, MA, USA). 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) (MTS) assay kit was purchased from Promega (Madison, WI, USA). Alkaline phosphatase (ALP) assay kit was obtained from Abcam (Cambridge, MA, USA). Alizarin red S (ARS) staining solution (40 mM) and cetylpyridinium chloride (CPC) were acquired from Sigma Aldrich Company (St. Louis, MO, USA). Cell lines and culture-related materials were purchased from Invitrogen (Carlsbad, CA, USA). All other chemicals if not mentioned were obtained from Sigma-Aldrich Company (St. Louis, MO, USA). All chemicals were analytical grade and consumed as received. Deionized water (D.I. H2O) and 0.10 M phosphate buffer saline (PBS) were used as main solvents throughout all experiments.
2.2. Fabrication and design of the PLA scaffolds.
All 3D printed patterns and constructs were designed through Solidworks 2016 software. The digital data sets were then saved as stereolithography (STL) files and proceeded by using Slic3r software to generate a set of G-code for 3D printing. Manual modification of G-code was added to better control the deposition of the spool PLA filament through a heated extrusion head (250 μm diameter) at 210 °C. A close collection distance (0.5–2 mm) enables the controllable deposition of melted PLA filament on a 40 °C collection surface affixed to the stage with X-Y-Z linear motion. Respective modulation of X, Y and Z motion generated various patterns of PLA filament in a layer-by-layer manner.
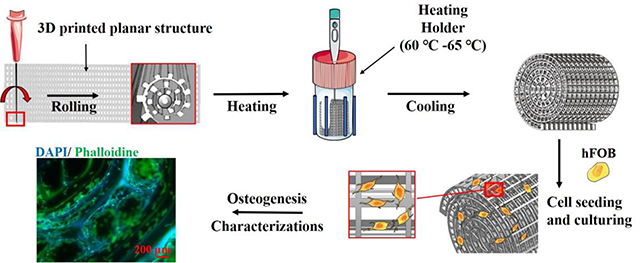
Three PLA scaffolds were designed and printed (MakerBot Replicator 3D Printer). For the porous cylinder and spiral scaffolds, the scaffolds were produced directly from the printer. However, for the porous spiral scaffold, a multi-layer plane structure was firstly printed through a conventional extrusion 3D printing approach and then rolled up by a 20G stainless steel needle to form the spiral porous construct. To be specific, the planar scaffold and needles were first preheated to decrease the rigidity of the whole constructs, and then the planar scaffold was slowly rolled up with the help of the needle into a spiral structure. After that, the compacted rolling scaffold was fixed into a temperature-tunable holder, which was preheated to about 65 °C to soften the printed filaments and enhance the adhesion between different layers. Finally, the holder would be quickly cooled down into room temperature for scaffold solidification. A schematic diagram of the experimental setup is described in Scheme 1.
Scheme 1.
Schematic diagram representing various stages of the fabrication of three different kinds of PLA specimen for bone tissue engineering.
2.3. PLA scaffold characterization.
Morphological analysis.
A Stereoscopic Zoom Microscope (Nikon SMZ1500) was used to visualize the architecture and morphology of the 3D scaffolds. The surface and internal morphology of the 3D scaffolds were imaged using a scanning electron microscope. In order to observe the inside of the scaffolds, the scaffolds were first frozen with liquid nitrogen for about 30 s, and then cut into halves with a razor. Finally, all the scaffolds (whether cut or not) were sputter-coated for 5 mins with a 2 nm Au layer, and the images were taken under an Auriga Modular Cross Beam workstation (Carl Zeiss, Inc.) at 3.00 kV.
Porosity measurement.
The porosity of the scaffolds (n=3) was measured by using the Archimedes’ principle in D.I. H2O. 34 The porosity was calculated according to the following equation:
Where Wsat stands for the weight of scaffold saturated with water, Wdry is the dry weight of the scaffold, and Wsus represents the weight of the scaffold suspended in water.
2.4. Mechanical testing.
A universal testing machine equipped with a 1000 N load cell (Chatillon, TCD 225 series) was used to estimate the compressive strength of the scaffolds. For each scaffold, three specimens were tested. In detail, scaffolds were loaded and tested at a speed of 0.5 mm/min, and the load-displacement data were collected every 10 ms. The stress-strain data were converted from the load-displacement data and the compressive modulus was found out from the slope of the stress-strain curve.
2.5. Cell culture and seeding.
Human fetal osteoblast (hFOB) cells were employed for cell culture studies. Briefly, hFOB cells were cultured in a 1:1 mixture of Ham’s F12 Medium and Dulbecco’s Modified Eagle’s Medium, supplemented with 2.5 mM L-glutamine, 1% penicillin/streptomycin, and 10% fetal bovine serum (FBS). The cells were kept in a humidified atmosphere with 5% CO2 at 37 °C, and the medium was changed after every 2 days of culture. The scaffolds were placed individually into a 24-well plate and sterilized by soaking in 70% ethanol for 2 h, washed triple times with sterile PBS before the cell study. During the cell seeding process, a concentration of 1×105 hFOB cells in 50 μL culture medium was loaded into a 100 μL syringe, and then the cells were slowly and gently added to the scaffold from all directions to ensure that the initial cell number and adhesion number were similar on different scaffolds. The cells were permitted to adhere in the incubator for 2 h, but due to the influence of gravity, the scaffold was flipped over every 20 mins to give the cells an equal chance of adhesion. 22 After 2 h incubation, each scaffold was transferred into a new 24-well plate and each well was filled with 1.5 mL of culture medium. The cultures were maintained for a total of 28 days, and the medium was changed every 2 days before the 14th day and every day after the 14th day.
2.6. Cell proliferation test.
The cell adhesion and proliferation of hFOB cells on different scaffolds were monitored on the 1st, 7th, 14th, 21th, and 28th days of culture using the MTS assay. To be specific, at the end of each culture period, the scaffolds (n=3) were changed to a new 24-well plate and washed twice with cold PBS. Afterward, each scaffold was added with 1.4 mL mixture containing 400 μL of MTS reagent and 1 mL culture medium. The plate was incubated in 5% CO2 at 37 °C for 2 h, and then 200 μL solution of each sample was shifted to a 96-well plate. The absorbance values were read by using a UV-vis microplate reader (BioTek Instruments, Inc., Vermont, USA) at 490 nm. Finally, the number of hFOB cells at each time point was calculated form the hFOB MTS standard curve (Figure S1).
2.7. ALP assay.
The osteoblast phenotype differentiation was measured by using the ALP assay kit according to the manufacture’s instruction after cell seeding for 14, 21, and 28 days. The process was as follows, at the end of each pre-designated time point, the scaffolds were taken out and washed with PBS for 3 times. Each scaffold (n=3) was then transferred to a 2 mL Eppendorf tube containing 1.5 mL assay buffer. The samples were centrifuged at 15000 rpm/min for 15 mins at 4 °C to remove any insoluble materials. Next, the supernatant was collected and transferred to a new Eppendorf tube. Then took a new 96-well plate and added the above supernatant to individual wells, 80 μl per well. 50 μl of 5 mM pNPP solution was poured to each well so that the total volume of each well was 130 μl. Afterward, the 96-well plate was kept in the dark at room temperature for incubation of about 1h, and then 20 μl of a stop solution was added to stop the reaction (the final volume of each well was 150 μl). At the same time, a new set of standards were needed to be prepared at each time point. Specifically, 10 μl of the ALP enzyme solution was added to 120 μl of standard pNPP wells of different concentrations and incubated for 1 h, and then 20 μl of the stop solution was added (the final volume of each well was also 150 μl). The absorbance was detected at 405 nm using a UV-vis microplate reader, and the ALP activity (U/mL) was calculated from the following equation:
Where the amount of pNP was calculated according to the standard trend line equation, the reaction time was set at 60 mins, the original sample volume was 80 μl, and the dilution factor was 1.
The final ALP activity was normalized by the cell number and expressed as U/ml/cell from a standard curve.
2.8. ARS staining and quantification.
The mineralized matrix deposition of the hFOB cells on the scaffolds was determined by ARS staining method, which can reveal the calcium deposition. After 14, 21, and 28 days of incubation, the scaffolds were fixed in 4% paraformaldehyde (PFA) for 1 h and washed with PBS three times. After that, each scaffold was stained with 1.5 mL ARS solution (40 mM) for 1 h under gentle shaking on a shaker. Next, the scaffolds were washed 5 times with D.I. H2O to remove excess ARS that did not attach to the scaffolds, and then the optical images (Nikon SMZ1500) were taken. To quantify the staining, each scaffold was immersed in 1.5 mL 10% (w/v) CPC 10 mM sodium phosphate solution overnight at room temperature to extract the stained ARS. The absorbance was measured at 562 nm using a UV-vis microplate reader and the concentration of ARS was calculated according to the standard curve, which dissolved the ARS in CPC with different concentrations. The concentration of ARS was finally normalized to cell number (mM/cell).
2.9. Cell adhesion and morphology on scaffolds.
In order to observe the morphology of the cells attached to the studied scaffolds, an immunofluorescence study was performed. Briefly, after cell seeding for 14, 21, and 28 days, the scaffolds were washed with cold PBS three times, fixed in 1.5 mL 4% PFA solution for 1 h. Next, the scaffolds were immersed in liquid nitrogen for 30 s and cut into halves from the middle part with a razor. The scaffolds were then transferred in 1.5 mL 0.1% TritonTM X-100 for 30 mins at room temperature and blocked with 1.5 mL 1% bovine serum albumin (BSA) solution in 10 mM PBS/20 mM glycine for 45 mins. Afterward, the scaffolds were immersed in Phalloidin (Alexa Fluor 488) solution for 1 h, and then the scaffolds were rinsed with PBS, stained with 4’,6-diamidino-2-phenylindole (DAPI) for 10 mins. The phalloidin was used to stain the actin filaments of the cytoskeleton with green color, and DAPI was used to dye the cells’ nuclei with blue color. Finally, the scaffolds were observed under a fluorescent microscope (Nikon Eclipse 80i epifluorescence).
2.10. Statistical analysis.
All the above data were presented as mean ± standard deviation and evaluated by the one-way ANOVA test. The results were considered statistically significant with a p-value less than 0.05.
3. Results and Discussion
3.1. Preparation and characterization of the 3D PLA scaffolds.
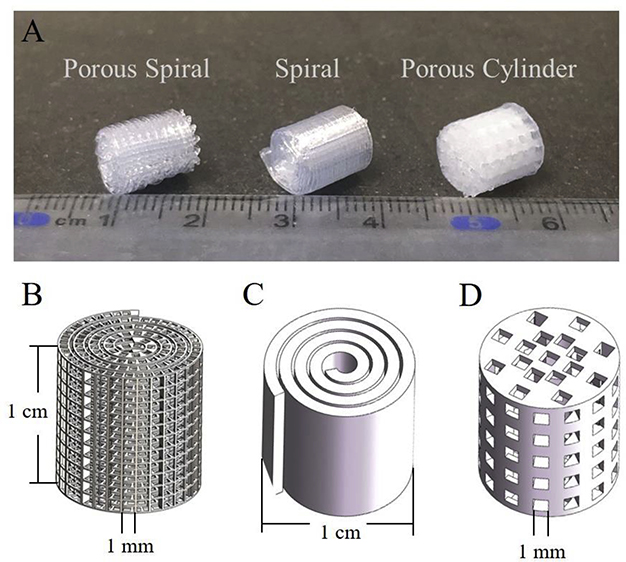
3D printing now allows the manufacture of complex scaffolds with different structures, pore sizes, porosities, and controllable orientation that were difficult to be achieved by traditional bio-fabrication methods. As shown in Figure 1B, the prototype designing of different constructs were created through Solidworks software. The porous spiral, spiral, and porous cylinder scaffolds were designed with 1 cm in height and 1 cm in diameter. The interval gaps between two parallel filaments for porous cylinder and porous spiral structure were designed as 1 mm. However, the minimal printed feature resolution of the extrusion printing method is generally over 100 μm due to the high-pressure resistance in the extrusion printing nozzle. Rapid and continuous move-stop transition highly limited the accuracy and printing resolution of current normal commercial 3D printers when forming the 3D construct with high porosity. Therefore, the porous cylinder scaffold having a low porosity can be easily produced by a 3D printer, but the current 3D printing method cannot obtain a high-quality and well-structured scaffold with high porosity, such as porous spiral scaffold. Instead of solely relying on the additive manufacturing 3D printing approach, we combined the conventional bio-fabrication method with the novel 3D printing (as shown in Scheme 1). Specifically, a 20G needle was used as a roller to be wrapped up to form the spiral structure with the demanding size. Various needle gauge substitutions allowed the flexibility of the central void dimension. In addition, the porosity and pore size can be easily tuned by adjusting the filament distance, shape and arrangement in a plane pattern. The post-treatment has proved that pre-heated PLA filament at 50 °C possessed the capacity and convenience for handling, and the whole printed structure could retain the stability after the cooling of filament. From the macroscopic view of all printed constructs (Figure 1A), the porous spiral could reach the same size as designed without losing the orientation of filament and pores arrangement.
Figure 1.
(A) Digital pictures of the porous spiral (left), spiral (middle), and porous cylinder (right) scaffolds printed by 3D-printer; Solidworks design for (B) porous spiral; (C) spiral; (D) porous cylinder scaffolds in a 3D view.
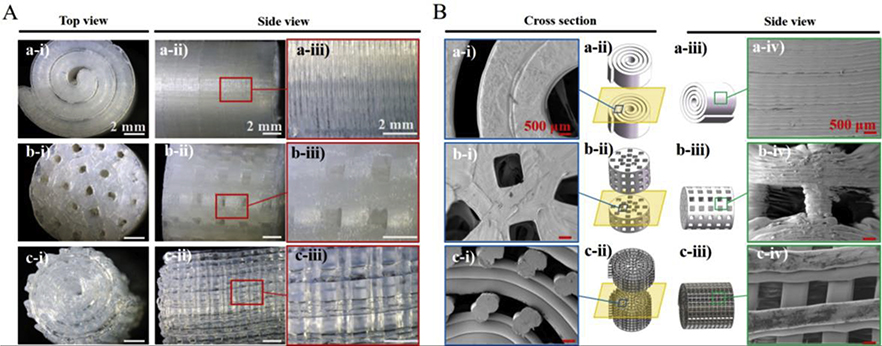
Further features were examined through the stereomicroscope (Figure 2A). In detail, it was observed from the top-view image that the diameter of the spiral scaffold was about 1 cm, which was consistent with the Solidworks design. Moreover, layers with a thickness of around 200 μm were seen to be stacked uniformly in the spiral scaffold from the side-view without any porous structure. Compared to the spiral scaffold, according to the optical images, the porous spiral exhibited regular arrays with interconnected pores which have an average diameter of about 920 μm, indicating highly regular pore formation and structure. For the porous cylinder scaffold, the average pore diameter on the top and side was around 950 μm and these pores were observed to be interconnected. From the top-view images, the measured dimensions of both porous scaffolds were about 1 cm. Interestingly, it was noticed that the pore sizes on both porous scaffolds were smaller than the designed model one (1000 μm). That was because of the spreading, thermal expansion, and subsequent cooling of the PLA matrix during the process of 3D printing.
Figure 2.
(A) Optical images of the spiral (top), porous cylinder (middle), and porous spiral (bottom) scaffolds showing the architecture from top view and side view. Scale bar: 2 mm; (B) Cross section view and side view SEM images of spiral (top), porous cylinder (middle), and porous spiral (bottom) scaffolds. Scale bar: 500 μm.
The presence of pore sizes, surface morphology, and pore interconnections of the scaffolds were further confirmed by the SEM images. The cross section-view and side-view of the scaffolds’ SEM images were presented in Figure 2B, and the cross-section-view could better illustrate the interconnection between different pores on the porous scaffolds. From both views, it is possible to observe well-defined geometry for all the PLA scaffolds similar to the optical images. The layer-by-layer stacking structure of the spiral scaffold can be more clearly observed in the SEM images. Moreover, all pores in the porous scaffolds were around 1 mm and were uniform, open, and interconnected with each other through a network of channels. More specifically, the pores on the porous cylinder scaffold were also stacked in a layer-by-layer manner, but the structure of the pores was not as uniform as the porous spiral scaffold. According to the previous publication, a large pore structure can support better bone formation because of improved cell implantation, new blood vessel infiltration, and high oxygenation. Based on early studies, Hulbert et al. 35 found out that the minimum requirements for a scaffold’s pore size were around 100 μm to satisfy bone growth. However, subsequent studies have shown that for scaffolds with apertures greater than 300 μm, have better osteogenesis. Small pores are good for hypoxic environments and induce osteochondral formation before osteogenesis, while well-vascularized macropores lead to osteogenesis directly without previous cartilage formation. Therefore, the pore sizes in these two porous scaffolds were suitable for bone regeneration.
In addition to pore sizes, an ideal porous scaffold for bone tissue regeneration should also have a high level of porosity and interconnected pore network. Generally, bone has a very complex structure. The cortical bone has a compact structure with a porosity of 3–12%. However, the porosity of trabecular is in the range of 50–90%, and the pore size is close to 1 mm. 36 The porosity of the three PLA scaffolds were measured by using the Archimedes’ principle in D.I. H2O. As shown in Table 1, the porosity of spiral, porous spiral, and porous cylinder were calculated to be 3.4±1.2%, 29.2±7.1%, and 69.3±7.4%, respectively. The spiral scaffold was solid without any pores but still exhibited a small porosity. That’s because the spiral structure could provide some space between the wall layers (Figure 2B). The porous spiral scaffold showed the highest porosity which was consistent with our hypothesis. As shown in Figure 2A, the porous cylinder scaffold shows about 6 pores in the enlarged image, while around 14 pores could be observed in the porous spiral scaffold. Similar results were also observed from the SEM images. We believed the higher porosity of porous spiral scaffold was due to its interconnected network and spiral structure. In addition to the porosity, these PLA scaffolds’ sample size, pore size, and surface area were listed in Table 1. Although only PLA material was applied in this study, the purpose was to demonstrate the stability of the materials after 3D printing. The contact angle values and the ATR-FTIR spectra of these PLA scaffolds were demonstrated in Figure S2.
Table 1.
Structural parameter and porosity of spiral, porous cylinder, and porous spiral scaffolds.
| Name | Size of sample | Macro pore size (mm) | Surface area (cm2) | Porosity % Experiment |
|---|---|---|---|---|
| Spiral | Diameter: 1 cm Height: 1 cm |
N/A | 0.7536 | 3.4±1.2 |
| Porous Cylinder | Diameter: 1 cm Height: 1 cm |
1±0.1 | 0.6358 | 29.2±7.1 |
| Porou Spiral | Diameter: 1 cm Height: 1 cm |
1±0.1 | 0.7522 | 69.3±7.4 |
N/A: not applicable.
3.2. Mechanical properties of the 3D PLA scaffolds.
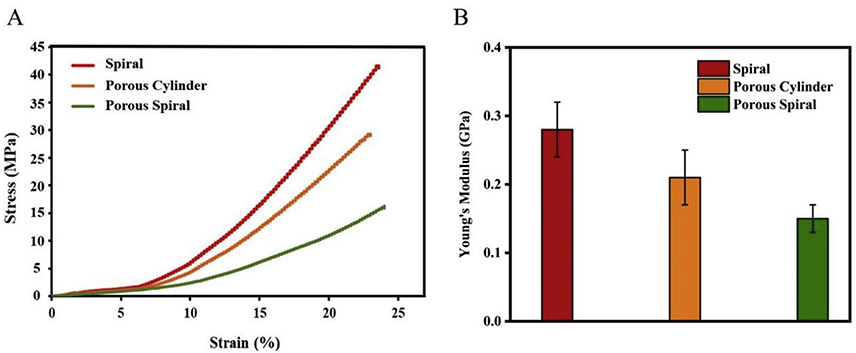
The mechanical properties of 3D structures are an important feature when considering the final application of the scaffolds. As shown in Figure 3A, the compressive stress of spiral, porous cylinder, and porous spiral scaffolds were found to be 41.38 MPa, 29.13 MPa, and 16.04 MPa, respectively. More specifically, the spiral scaffold had the maximum compressive stress increased by 29.61% and 61.23% when compared with porous cylinder and porous spiral. The highest compressive stress of the spiral scaffold was due to its solid structure. When the load was applied parallel to the stacking direction, layers were strongly connected to each other to increase the mechanical strength. In contrast, the porosity in the scaffolds causes a reduction in mechanical properties because it impairs the structural integrity of the scaffold, which as a result will not be suitable for load bearing. 37 Generally, the higher the percentage of porosity, the lower the mechanical strength will be. In addition, the compression modulus was calculated from the slope of the linear portion of the Stress-Strain curve. Figure 3B exhibits the data corresponding to the Young’s modulus for the spiral, porous cylinder, and porous spiral scaffolds. Like the compressive stress, spiral scaffold shows the largest Young’s modulus of 0.28±0.04 GPa, while porous spiral with the highest porosity shows the smallest Young’s modulus of 0.15±0.02 GPa. Although porous spiral scaffold present lower compressive stress and Young’s modulus, it is still appropriate for the bone regeneration. This is because the typical compressive stress of cancellous bone ranges from 0.2 to 80 MPa and its Young’s modulus is in the range of 0.01 to 0.2 Gpa. 38 The values obtained from our study (16.04 MPa and 0.15±0.02 GPa) are in agreement with the aforementioned literature findings, which clearly indicate that the porous spiral scaffold would be a suitable candidate for this application.
Figure 3.
The compressive stress/strain representative curves (A) and Young’s modulus (n = 3) of spiral (red), porous cylinder (orange), and porous spiral (green) scaffolds.
3.3. hFOB cell proliferation on the 3D PLA scaffolds.
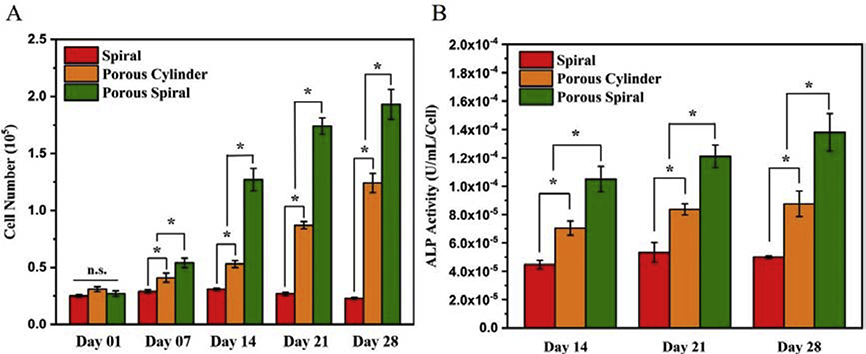
As shown in Figure 4A, the viabilities of the hFOB cells were quantitatively measured by MTS assay after culturing for up to 28 days. The hFOB cell is an immortalized clonal human fetal cell line with good characteristics and can be used as a osteoprogenitor with minimal karyotype damage even after multiple passages. 39 The intensity of MTS was directly related to the number of viable cells on the scaffolds, and the cell proliferation was shown to increase with culturing time on all the scaffolds. To be specific, spiral, porous cylinder, and porous spiral scaffolds exhibited similar cell viabilities after 1 day of culture. The results presented that hFOB cells initially adhered to these three scaffolds in similar amounts, providing an excellent platform for cell culture. However, on day 7, the cell viabilities on porous scaffolds were significantly higher than that on the spiral scaffold, and the porous spiral scaffold showed the highest viability. By extending the culture time up to 14 days, the cell number on the porous spiral remained increased to 1.27×105, which was about 2.4 times and 4.1 times higher than that of porous cylinder and spiral scaffolds, respectively. This growth trend continued until the 28th day of culture, in which the cell number on spiral, porous cylinder, and porous spiral scaffolds reached 2.3×104, 1.24×105, and 1.93×105, respectively. The cell proliferation was also assessed using the Quant-iT™ PicoGreen® dsDNA Assay Kit (Figure S3). Both the MTS and PicoGreen results demonstrated that, compared with solid scaffold, porous scaffold played an important role in supporting cell attachment and proliferation. That was because the cell culture could be strongly influenced by the supporting scaffold structure. All the three scaffolds were produced from PLA material without any modification. However, porous cylinder and porous spiral scaffolds had more open and interconnected pore structure to induce cell ingrowth within the scaffold. Moreover, the porous and spiral structure could provide a larger surface area to volume ratio, which enhanced the adsorption of proteins from the culture medium, thereby improving cell attachment and spreading. Since the porosity and surface area of porous spiral scaffold was much larger than that of the porous cylinder scaffold, hFOB cells seeded on porous spiral scaffold had more space to grow and proliferate, and thus the MTS results showed the highest cell viability. Conversely, as shown in Figure 4A, the cell proliferation on the solid spiral scaffold did not increase significantly as the culture time prolonged. We believed this was because the surface of the spiral scaffold was smooth and curved. When seeding and culturing the cells, due to the static culture system, the cells would flow downward because of the potential energy of gravity and cause poor cell attachment and cell aggregation at the bottom of the plates. 40 In addition, PLA is a hydrophobic polymer with weak cell affinity. Therefore, cells are more willing to grow on culture plates than on PLA material. However, even in the static culture system, porous PLA scaffolds could provide more open structures and growth areas to capture and induce cell ingrowth. This further illustrated the importance of porous structures for bone regeneration of PLA materials.
Figure 4.
(A) MTS assay showing hFOB cells attachment and proliferation on spiral, porous cylinder, and porous spiral scaffolds for 1, 7, 14, 21, and 28 days; (B) Alkaline phosphatase (ALP) activity secreted by hFOB cells cultured on spiral, porous cylinder, and porous spiral scaffolds for 14, 21, and 28 days (*: P < 0.05; n.s.: p > 0.05 no significant difference).
3.4. Osteogenic differentiation.
As an early indicator of the osteogenic development of the hFOB cells, 41 alkaline phosphatase (ALP) activity was measured after 14, 21, and 28 days of culture, and the results were shown in Figure 4B. In general, the ALP activity of three PLA scaffolds gradually increased with time, and the ALP value of the porous spiral scaffold was significantly higher than that of the other two scaffolds at all the culture time points studied, which was similar to the results of the MTS assay. To be specific, after 14 days of incubation, the ALP values of the porous cylinder and porous spiral scaffolds were 7.05×10−5 and 1.08×10−4, which were significantly greater than the ALP value of spiral scaffold at 4.48×10−5. On day 14, the difference between three scaffolds was already apparent, but on day 21 the difference was even greater. In particular, the level of ALP on the porous spiral scaffold was approximately 1.4 and 2.3 times higher than that on the porous cylinder and solid spiral scaffolds, respectively. From day 21 to day 28, the ALP level of hFOB cells grown on all the three scaffolds did not increase a lot, indicating that most osteoblast differentiation and bone matrix formation were completed on day 21. These results indicated that porous scaffold could promote the ALP activity for the osteogenic differentiation of hFOB cells. This was due to the higher porosity and larger surface area of the porous scaffold, which could first enhance the cell proliferation and then further promote cell differentiation compared to non-porous scaffold, even when the initial cell attachment number was similar. In addition to the structure of the scaffold, PLA also played a key role in increasing ALP activity. The previous studies have suggested that higher mechanical strength might provide better mechanical support for cell growth and differentiation by supplying enough oxygen and nutrient exchanges, which was considered as a major challenge for cell culture in thick 3D scaffolds. According to the above results, both porous cylinder and porous spiral scaffolds have good mechanical strength to support osteogenic differentiation of hFOB cells. Although spiral scaffold exhibited the highest mechanical strength, the ALP activity was the lowest among all scaffolds due to limited cell attachment. Moreover, PLA is a biodegradable polymer and its degradation byproducts may induce cytotoxic response. However, the increased hFOB cell proliferation and differentiation results indicated that due to the slow degradation rate, the cytotoxicity of the PLA byproducts was negligible. Recently, researchers have found out that the degradation products of PLA might also help enhance osteogenic differentiation and bone formation on scaffolds. Lactate, a major degradation product of PLA, is known for its ability to stimulate collagen synthesis and new vessel formation. 42 In summary, these results demonstrated that porous spiral scaffold with large surface area, high porosity, good mechanical strength, and slow degradation rate could significantly enhance the osteogenic differentiation of hFOB cells.
3.5. ARS staining and quantification.
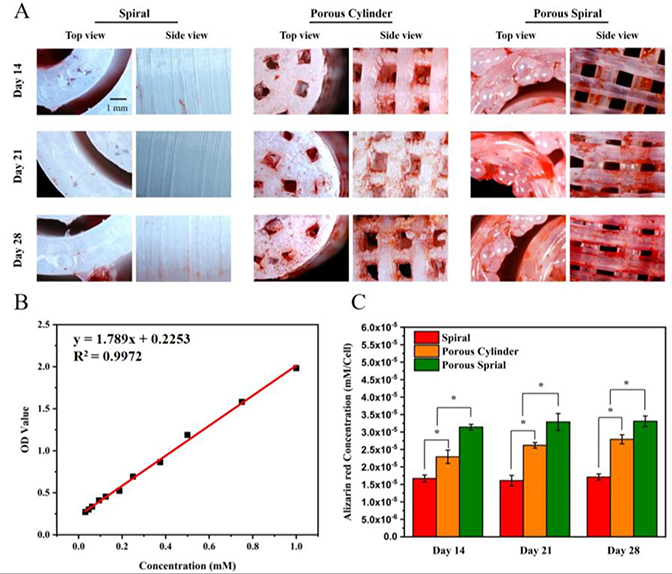
The production of calcium deposit at the later stage of differentiation was another key indicator for osteogenic efficiency of hFOB cells. 43 This mineralization was studied by Alizarin red S (ARS) staining method after 14, 21, and 28 days of cell culture. Figure 5A shows the microscopic ARS stained images of different PLA scaffolds from both top-view and side-view. The red stains were ARS-calcium chelating product indicating the presence of calcium. From day 14 to day 28, very little and pale red staining was observed on the spiral scaffold, indicating that the calcium deposition on this scaffold was very limited. In comparison, the cells cultured on porous cylinder scaffold exhibited brighter red staining than the spiral scaffold throughout the culture time. To be specific, on the 14th day, there was little mineral staining on the porous cylinder scaffold, on the 21st day a noticeable mineral deposition was observed, and on the 28th day, the mineral staining increased greatly. However, hFOB cells on porous spiral scaffold showed more intense ARS staining than that on the porous cylinder scaffold at all the time points studied, and exhibited the highest production of calcium nodules among all the groups. Specifically, on day 14, there was a large and intense ARS red area in the porous spiral scaffold, and the area and color increased and deepened over time. In order to quantify the mineralization, scaffolds were desorbed with 10% CPC overnight, and the absorbance of the solution was measured at 562 nm. The results of the quantitative analysis of ARS staining were shown in Figure 5C. It could be clearly observed that the absorbance of the solution eluted from the porous scaffolds on day 14 was significantly higher than that of the solution eluted from the solid spiral scaffold. On day 21, the ARS intensity of porous cylinder and porous spiral scaffolds showed a 1.6-fold and 2.1-fold increase, respectively, compared to the spiral scaffold. On day 28, this difference did not change much compared with the results of 21 days, which remained at around 1.7-fold and 2.0-fold. In summary, both the qualitative and quantitative analysis of calcium expression exhibited that porous spiral scaffold effectively improved the formation and calcification of the bone matrix. In the hFOB cells mineralization process, the unique structure of the porous spiral scaffold (such as porosity, pore size, and surface area) can enhance the cell proliferation, while the mechanical strength of the porous spiral scaffold and the properties of PLA itself can promote mineral deposition. This synergistic effect allows hFOB cells to effectively adhere, proliferate, and mineralize to mimic the ECM and is suitable for bone tissue regeneration.
Figure 5.
(A) Assessment of mineralization by Alizarin red S (ARS) staining of the cell seeded on spiral, porous cylinder, and porous spiral scaffolds for 14, 21, and 28 days; (B) The standard curve of ARS in cetylpyridinium chloride for quantification; (C) Calcium deposition quantified by ARS assay extracted from day 14, 21 and 28 (*: P < 0.05).
3.6. Immunofluorescence images of hFOB on 3D PLA scaffolds.
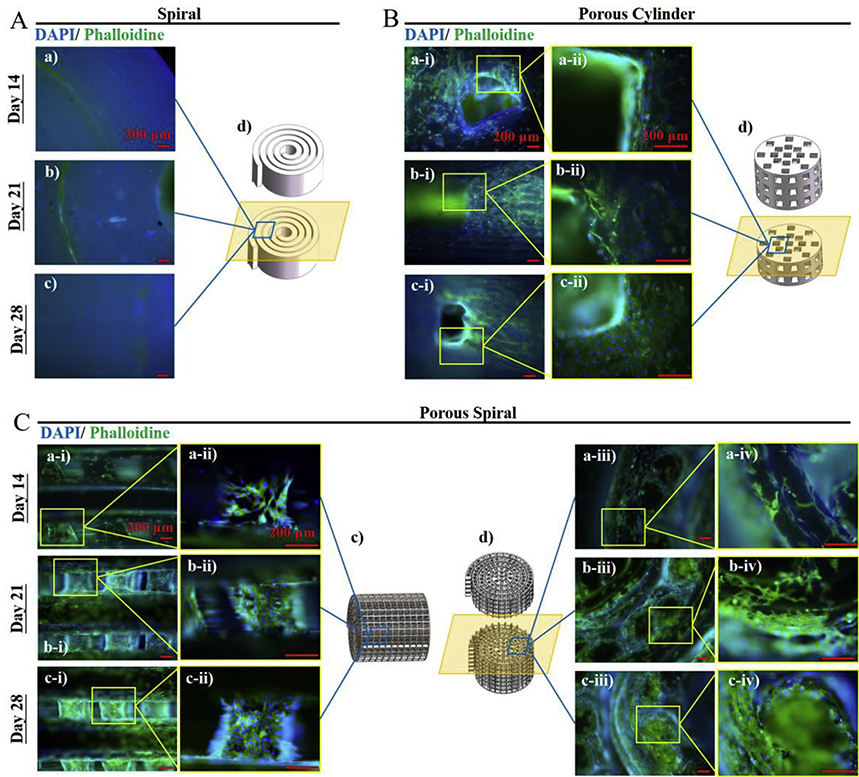
In addition to cell viabilities, the adhesion and morphology of hFOB cells in the three PLA scaffolds were also observed by immunofluorescence images after culturing for 14, 21, and 28 days. 44 In the fluorescence microscopy images (Figure 6), the nucleus of living cells were stained with DAIP (blue) and the active cytoplasm was stained with Phalloidin (green). In order to observe the cell growth inside the scaffolds, the scaffolds were cut into halves and the zoom-in fluorescent images were taken. After culturing for 14 days, most hFOB cells were spread throughout the entire interior of the two porous scaffold strands and no distinguishable differences were observed between the cell morphologies. It could also be observed that the cells had begun to fill the interconnected pore network of the porous scaffolds. After 1 week, the interior of both porous scaffolds were covered with cells and the cells had grown in the pores of the scaffolds, but it was not possible to observe from the images which scaffold had more cells. However, from previous MTS results, it could be seen that more cells were proliferated on the porous spiral scaffold than the porous cylinder scaffold. On day 28, the fluorescent images showed that hFOB cells could colonize on the surface and inside of the porous scaffolds, and form a cell-to-cell and cell-to-ECM interconnected network throughout the entire 3D porous structure. In contrast, almost no living cells were observed inside the solid spiral scaffold throughout the time period. This result was in consistent with the MTS results, indicating that when there was no porous structure, the cells were hard to grow on curved and smooth PLA material in the static culture system. In conclusion, it could be seen from the fluorescent images that both the porous spiral scaffold and the porous cylindrical scaffold presented excellent cell compatibility due to their unique structures and could potentially be applied to bone regeneration.
Figure 6.
Fluorescent images of hFOB cells cultured on (A) spiral, (B) porous cylinder, and (C) porous spiral scaffolds for 14, 21, and 28 days. The cells were stained by DAPI (blue) and Phalloidin (green). Scale Bar: 200 μm.
4. Conclusion
In this study, we designed and fabricated the PLA porous cylinder, porous spiral, and solid spiral scaffolds using the 3D printing method. Both porous scaffolds have an average pore size of 930 nm and all pores were interconnected. The pores were large enough to improve cell implantation, new blood vessel infiltration, and high oxygenation. The porous cylinder scaffold with low porosity (around 30%) could be fabricated directly from the printer. However, in order to prepare high-porosity scaffold (porous spiral scaffold), we combined the traditional bio-fabrication method with the novel 3D printing, because the current 3D printing method cannot obtain a high-quality and well-structured scaffold with high porosity. The compressive properties of both porous scaffolds were found to be appropriate within the range of human cancellous bone. The attachment and proliferation of hFOB cells were significantly greater on the porous scaffolds than that of the solid spiral scaffold, and porous spiral scaffold was the most favorable for cell growth due to its more open and interconnected pore structure to induce cell ingrowth. Furthermore, the enhanced ALP activity and mineral deposition suggested that porous spiral scaffold could stimulate new bone formation better than the other two scaffolds. In summary, the combination of traditional bio-fabrication and 3D printing method has produced the porous spiral scaffold with unique structure, mechanical stability, and stimulation of cell proliferation and differentiation for bone tissue applications.
Supplementary Material
Highlights.
The PLA porous spiral (PPS) scaffold is prepared for bone tissue regeneration.
The PPS scaffold can improve the internal connections of porous networks.
The PPS scaffold shows high mechanical strength.
The PPS scaffold can induce better osteogenesis and mineralization for hFOB cells.
Acknowledgments
Funding Sources
This study was partly supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health (award number R01EB020640), and by the U.S. Army Medical Research Acquisition Activity (USAMRAA), through the CDMRP Peer Reviewed Medical Research Program under Award No. W81XWH2010321.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amini AR; Laurencin CT; Nukavarapu SP, Bone Tissue Engineering: Recent Advances and Challenges. Critical Reviews in Biomedical Engineering 2012, 40 (5), 363–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burg KJ; Porter S; Kellam JF, Biomaterial Developments for Bone Tissue Engineering. Biomaterials 2000, 21 (23), 2347–59. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg VM; Stevenson S, Natural History of Autografts and Allografts. Clin Clinical Orthopaedics and Related Research 1987, (225), 7–16. [PubMed] [Google Scholar]
- 4.Pelker RR; Friedlaender GE, Biomechanical Aspects of Bone Autografts and Allografts. Orthopopedic Clinics of North America 1987, 18 (2), 235–239. [PubMed] [Google Scholar]
- 5.Liu X; Ma PX, Polymeric Scaffolds for Bone Tissue Engineering. Annals of Biomedical Engineering 2004, 32 (3), 477–486. [DOI] [PubMed] [Google Scholar]
- 6.Bose S; Roy M; Bandyopadhyay A, Recent Advances in Bone Tissue Engineering Scaffolds. Trends in Biotechnology 2012, 30 (10), 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rakovsky A; Gotman I; Rabkin E; Gutmanas EY, β-TCP–polylactide Composite Scaffolds with High Strength and Enhanced Permeability Prepared by a Modified Salt Leaching Method. Journal of the Mechanical Behavior of Biomedical Materials 2014, 32, 89–98. [DOI] [PubMed] [Google Scholar]
- 8.Kim HJ; Park IK; Kim JH; Cho CS; Kim MS, Gas Foaming Fabrication of Porous Biphasic Calcium Phosphate for Bone Regeneration. Tissue Engineering and Regenerative Medicine 2012, 9 (2), 63–68. [Google Scholar]
- 9.Thadavirul N; Pavasant P; Supaphol P, Development of Polycaprolactone Porous Scaffolds by Combining Solvent Casting, Particulate Leaching, and Polymer Leaching Techniques for Bone Tissue Engineering. Journal of Biomedical Materials Research Part A 2014, 102 (10), 3379–3392. [DOI] [PubMed] [Google Scholar]
- 10.Akbarzadeh R; Yousefi A-M, Effects of Processing Parameters in Thermally Induced Phase Separation Technique on Porous Architecture of Scaffolds for Bone Tissue Engineering. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2014, 102 (6), 1304–1315. [DOI] [PubMed] [Google Scholar]
- 11.Reves BT; Bumgardner JD; Cole JA; Yang Y; Haggard WO, Lyophilization to Improve Drug Delivery for Chitosan-calcium Phosphate Bone Scaffold Construct: A Preliminary Investigation. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2009, 90B (1), 1–10. [DOI] [PubMed] [Google Scholar]
- 12.Park SA; Lee SH; Kim WD, Fabrication of Porous Polycaprolactone/hydroxyapatite (PCL/HA) Blend Scaffolds using a 3D Plotting System for Bone Tissue Engineering. Bioprocess and Biosystems Engineering 2011, 34 (4), 505–13. [DOI] [PubMed] [Google Scholar]
- 13.Placone JK; Engler AJ, Recent Advances in Extrusion-Based 3D Printing for Biomedical Applications. Advanced Healthcare Materials 2018, 7 (8), e1701161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergmann C; Lindner M; Zhang W; Koczur K; Kirsten A; Telle R; Fischer H, 3D Printing of Bone Substitute Implants using Calcium Phosphate and Bioactive Glasses. Journal of the European Ceramic Society 2010, 30 (12), 2563–2567. [Google Scholar]
- 15.Kondiah PPD; Choonara YE; Kondiah PJ; Marimuthu T; du Toit LC; Kumar P; Pillay V, 3 - Recent Progress in 3D-printed Polymeric Scaffolds for Bone Tissue Engineering. In Advanced 3D-Printed Systems and Nanosystems for Drug Delivery and Tissue Engineering, 2020, pp 59–81. [Google Scholar]
- 16.Jackson RJ; Patrick PS; Page K; Powell MJ; Lythgoe MF; Miodownik MA; Parkin IP; Carmalt CJ; Kalber TL; Bear JC, Chemically Treated 3D Printed Polymer Scaffolds for Biomineral Formation. ACS Omega 2018, 3 (4), 4342–4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X; Holzwarth JM; Ma PX, Functionalized Synthetic Biodegradable Polymer Scaffolds for Tissue Engineering. Macromolecular Bioscience 2012, 12 (7), 911–919. [DOI] [PubMed] [Google Scholar]
- 18.Gregor A; Filová E; Novák M; Kronek J; Chlup H; Buzgo M; Blahnová V; Lukášová V; Bartoš M; Nečas A; Hošek J, Designing of PLA Scaffolds for Bone Tissue Replacement Fabricated by Ordinary Commercial 3D printer. Journal of Biological Engineering 2017, 11 (1), 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garlotta D, A Literature Review of Poly(Lactic Acid). Journal of Polymers and the Environment 2001, 9 (2), 63–84. [Google Scholar]
- 20.Rodrigues N; Benning M; Ferreira AM; Dixon L; Dalgarno K, Manufacture and Characterisation of Porous PLA Scaffolds. Procedia CIRP 2016, 49, 33–38. [Google Scholar]
- 21.Rosenzweig DH; Carelli E; Steffen T; Jarzem P; Haglund L, 3D-Printed ABS and PLA Scaffolds for Cartilage and Nucleus Pulposus Tissue Regeneration. International Journal of Molecular Sciences 2015, 16 (7), 15118–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fairag R; Rosenzweig DH; Ramirez-Garcialuna JL; Weber MH; Haglund L, Three-Dimensional Printed Polylactic Acid Scaffolds Promote Bone-like Matrix Deposition in Vitro. ACS Applied Materials & Interfaces 2019, 11 (17), 15306–15315. [DOI] [PubMed] [Google Scholar]
- 23.Kao C-T; Lin C-C; Chen Y-W; Yeh C-H; Fang H-Y; Shie M-Y, Poly(dopamine) coating of 3D Printed Poly(lactic acid) Scaffolds for Bone Tissue ngineering. Materials Science and Engineering: C 2015, 56, 165–173. [DOI] [PubMed] [Google Scholar]
- 24.Kapat K; Srivas PK; Rameshbabu AP; Maity PP; Jana S; Dutta J; Majumdar P; Chakrabarti D; Dhara S, Influence of Porosity and Pore-Size Distribution in Ti6Al4 V Foam on Physicomechanical Properties, Osteogenesis, and Quantitative Validation of Bone Ingrowth by Micro-Computed Tomography. ACS Applied Materials & Interfaces 2017, 9 (45), 39235–39248. [DOI] [PubMed] [Google Scholar]
- 25.Cheng MQ; Wahafu T; Jiang GF; Liu W; Qiao YQ; Peng XC; Cheng T; Zhang XL; He G; Liu XY, A Novel Open-porous Magnesium Scaffold with Controllable Microstructures and Properties for Bone Regeneration. Scientific Report 2016, 6, 24134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abbasi N; Hamlet S; Love RM; Nguyen N-T, Porous Scaffolds for Bone Regeneration. Journal of Science: Advanced Materials and Devices 2020. [Google Scholar]
- 27.Bhattacharjee N; Urrios A; Kang S; Folch A, The Upcoming 3D-printing Revolution in Microfluidics. Lab on a Chip 2016, 16 (10), 1720–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chia HN; Wu BM, Recent Advances in 3D Printing of Biomaterials. Journal of Biological Engineering 2015, 9 (1), 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohanty S; Sanger K; Heiskanen A; Trifol J; Szabo P; Dufva M; Emnéus J; Wolff A, Fabrication of Scalable Tissue Engineering Scaffolds with Dual-pore Microarchitecture by Combining 3D Printing and Particle Leaching. Materials Science and Engineering: C 2016, 61, 180–189. [DOI] [PubMed] [Google Scholar]
- 30.Jakus AE; Geisendorfer NR; Lewis PL; Shah RN, 3D-printing Porosity: A New Approach to Creating Elevated Porosity Materials and Structures. Acta Biomaterialia 2018, 72, 94–109. [DOI] [PubMed] [Google Scholar]
- 31.Xia H; Zhao D; Zhu H; Hua Y; Xiao K; Xu Y; Liu Y; Chen W; Liu Y; Zhang W; Liu W; Tang S; Cao Y; Wang X; Chen HH; Zhou G, Lyophilized Scaffolds Fabricated from 3D-Printed Photocurable Natural Hydrogel for Cartilage Regeneration. ACS Applied Materials & Interfaces 2018, 10 (37), 31704–31715. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X; Chang W; Lee P; Wang Y; Yang M; Li J; Kumbar SG; Yu X, Polymer-Ceramic Spiral Structured Scaffolds for Bone Tissue Engineering: Effect of Hydroxyapatite Composition on Human Fetal Osteoblasts. PLOS ONE 2014, 9 (1), e85871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manoukian OS; Aravamudhan A; Lee P; Arul MR; Yu X; Rudraiah S; Kumbar SG, Spiral Layer-by-Layer Micro-Nanostructured Scaffolds for Bone Tissue Engineering. ACS Biomaterials Science & Engineering 2018, 4 (6), 2181–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J; Zhao S; Zhu Y; Huang Y; Zhu M; Tao C; Zhang C, Three-dimensional Printing of Strontium-containing Mesoporous Bioactive Glass Scaffolds for Bone Regeneration. Acta Biomaterialia 2014, 10 (5), 2269–2281. [DOI] [PubMed] [Google Scholar]
- 35.Hulbert SF; Young FA; Mathews RS; Klawitter JJ; Talbert CD; Stelling FH, Potential of Ceramic Materials as Permanently Implantable Skeletal Prostheses. Journal of Biomedical Materials Research 1970, 4 (3), 433–56. [DOI] [PubMed] [Google Scholar]
- 36.Kasten P; Beyen I; Niemeyer P; Luginbühl R; Bohner M; Richter W, Porosity and Pore Size of β-tricalcium Phosphate Scaffold can Influence Protein Production and Osteogenic Differentiation of Human Mesenchymal Stem Cells: An in Vitro and in Vivo Study. Acta Biomaterialia 2008, 4 (6), 1904–1915. [DOI] [PubMed] [Google Scholar]
- 37.Le Huec JC; Schaeverbeke T; Clement D; Faber J; Le Rebeller A, Influence of Porosity on the Mechanical Resistance of Hydroxyapatite Ceramics under Compressive Stress. Biomaterials 1995, 16 (2), 113–118. [DOI] [PubMed] [Google Scholar]
- 38.Rohl L; Larsen E; Linde F; Odgaard A; Jorgensen J, Tensile and Compressive Properties of Cancellous Bone. Journal of Biomechanics 1991, 24 (12), 1143–9. [DOI] [PubMed] [Google Scholar]
- 39.Yen M.-l.; Chien C-C; Chiu I.-m.; Huang H-I; Chen Y-C; Hu H-I; Yen BL, Multilineage Differentiation and Characterization of the Human Fetal Osteoblastic 1.19 Cell Line: A Possible in Vitro Model of Human Mesenchymal Progenitors. Stem Cells 2007, 25 (1), 125–131. [DOI] [PubMed] [Google Scholar]
- 40.Zhou G; Chang W; Zhou X; Chen Y; Dai F; Anwar A; Yu X, Nanofibrous Nerve Conduits with Nerve Growth Factors and Bone Marrow Stromal Cells Pre-Cultured in Bioreactors for Peripheral Nerve Regeneration. ACS Applied Materials & Interfaces 2020, 10.1021/acsami.0c04191. [DOI] [PubMed] [Google Scholar]
- 41.Clarke S; Choi SY; McKechnie M; Burke G; Dunne N; Walker G; Cunningham E; Buchanan F, Osteogenic Cell Response to 3-D Hydroxyapatite Scaffolds Developed via Replication of Natural Marine Sponges. Journal of Materials Science: Materials in Medicine 2016, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao Q; Cosme JG; Xu T; Miszuk JM; Picciani PH; Fong H; Sun H, Three Dimensional Electrospun PCL/PLA Blend Nanofibrous Scaffolds with Significantly Improved Stem Cells Osteogenic Differentiation and Cranial Bone Formation. Biomaterials 2017, 115, 115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prabhakaran MP; Venugopal J; Ramakrishna S, Electrospun Nanostructured Scaffolds for Bone Tissue Engineering. Acta Biomaterialia 2009, 5 (8), 2884–2893. [DOI] [PubMed] [Google Scholar]
- 44.Thi Hiep N; Chan Khon H; Dai Hai N; Byong-Taek L; Van Toi V; Thanh Hung L, Biocompatibility of PCL/PLGA-BCP Porous Scaffold for Bone Tissue Engineering Applications. Journal of biomaterials science. Polymer edition 2017, 28 (9), 864–878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.