Figure 5. The rate of actin delivery depends on the distance between profilin and the barbed end.
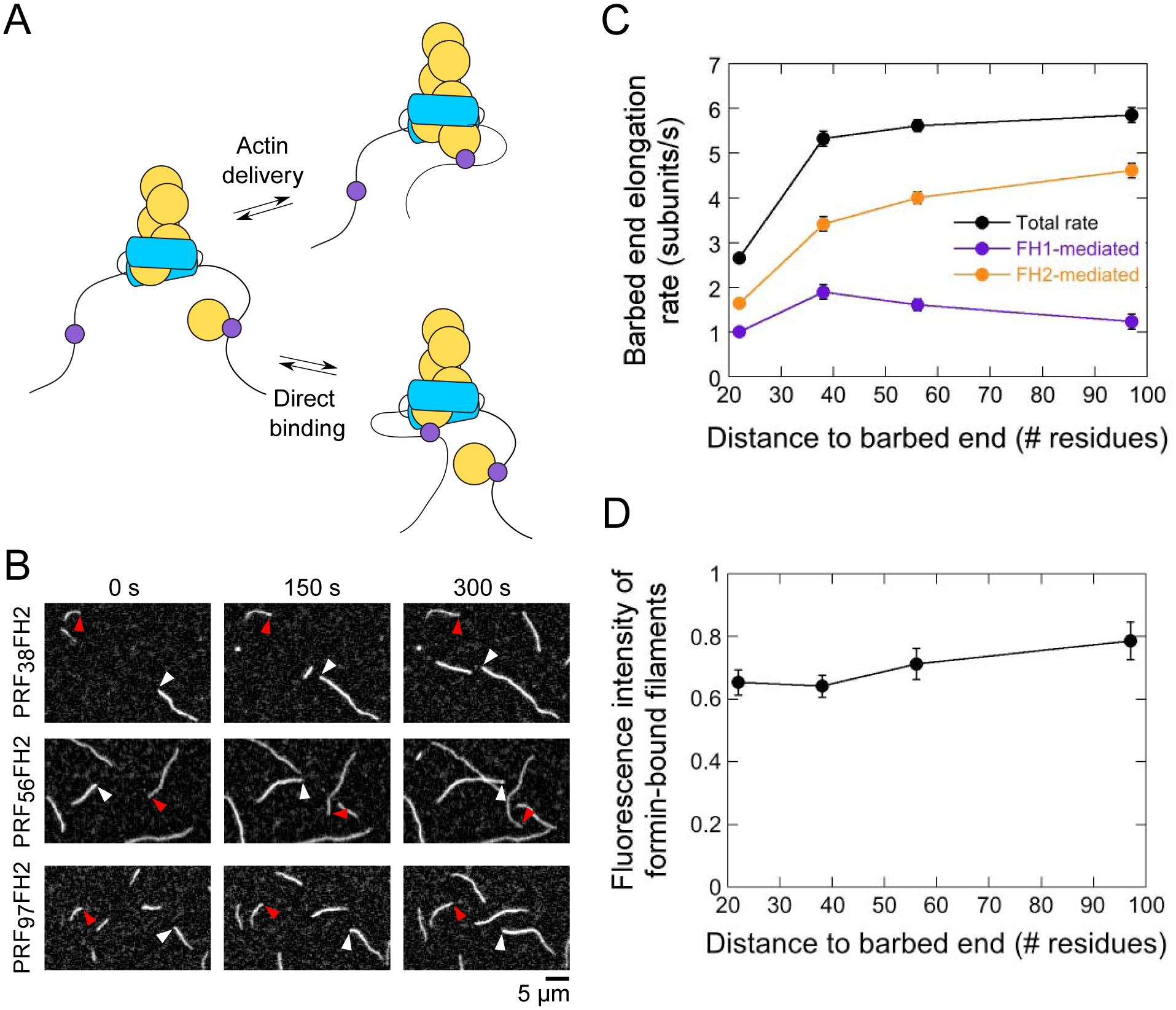
The experimental conditions were as follows: 0.75 μM actin (33% Oregon Green-labeled) in microscopy buffer. The data were collected by TIRF microscopy. (A) FH1-tethered profilin can bind the barbed end either via FH1-mediated actin delivery (upper reaction scheme) or via a direct binding event (lower reaction scheme). (B) Time series of TIRF micrographs of actin filament elongation in the absence and presence of PRF38FH2, PRF56FH2 and PRF97FH2. White and red arrowheads indicate the barbed ends of free and formin-bound actin filaments, respectively. (C) Dependence of the total (black data), FH1-mediated (purple data) and FH2-mediated (orange data) barbed end elongation rates mediated by the PRFxxFH2 constructs on the number of residues separating the profilin and Bni1p’s FH2 domain. Error bars are standard errors of the mean elongation rates measured for at least 15 formin-bound filaments. (D) Dependence of the fluorescence intensities of actin filaments polymerized by the Bni1p PRFxxFH2 constructs on the number of residues separating the profilin and Bni1p’s FH2 domain. Fluorescence intensities of formin-bound filaments were normalized to the intensities of filaments that are not formin-bound. Error bars are standard errors of the mean intensities of at least 15 filaments.
