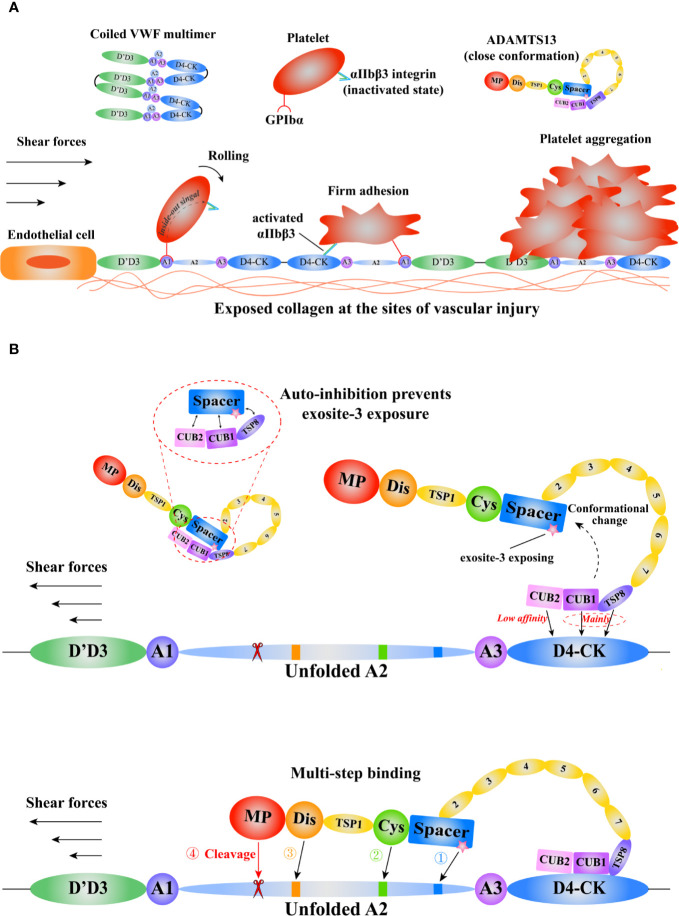
Figure 2.
A schematic diagram of the mechanisms of VWF and ADAMTS13 in thrombus formation. Adapted from Petri et al. (64). (A) The function of VWF in thrombosis. The coiled VWF multimers tether to the exposed subendothelial collagen at sites of vascular injury. Then, the globular VWF is gradually stretched into an elongated state under tension. Subsequently, the platelet receptor GPIbα binds to the VWF A1domain to induce the rolling of platelet, and activate the integrin αIIbβ3 that interacts with D4-CK domains resulting in firm adhesion, promoting platelet aggregation. (B) The model of VWF-mediated conformational change of ADAMTS13 and subsequent multi-step binding process. The circulating ADAMTS13 in a closed conformation prevents the exposure of the exosite-3 in Spacer domain. This auto-inhibition of ADAMTS13 is relieved mainly through the interactions between ADAMTS13 TSP8-CUB1 domains and VWF D4-CK domains. Then, the high shear forces and flexible TSP type 1 repeats (T1-T8) assist the “open” ADAMTS13 to get close to the unfolded VWF A2 domain. After a multi-step binding, the MP domain recognizes and cleaves the scissile bond Y1605-M1606 in A2 domain. VWF, von Willebrand Factor; GPIbα, platelet glycoprotein Ibα; ADAMTS13, a disintegrin-like and metalloproteinase with thrombospondin type-1 motifs, member 13; TSP, thrombospondin; CUB, complement C1r/C1s, Uegf (epidermal growth factor–related sea urchin protein), and Bmp1 (bone morphogenetic protein 1); MP, metalloproteinase.