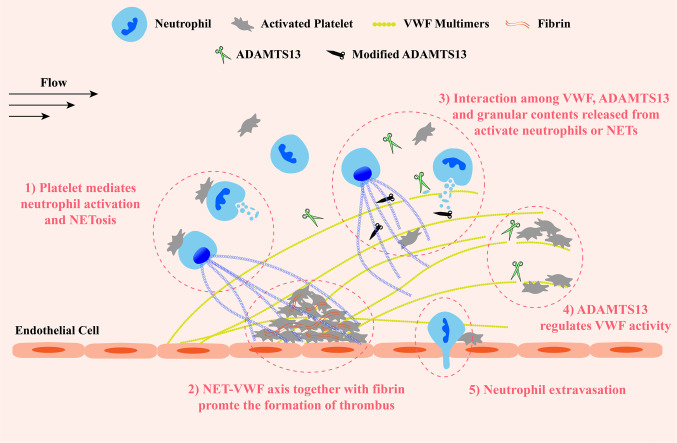
Figure 3.
A schematic diagram of the VWF-NET axis in thrombus formation and tissue damage at sites of vascular injury. Upon damage, VWF multimers are released from endothelial cells and capture circulating platelets to injury sites. VWF multimers recruit blood cells including neutrophils and erythrocytes. ADAMTS13 cleaves excessive VWF multimers to regulate their activity preventing the growth of thrombus. 1) The interaction among neutrophils, platelets, and VWF forms a vicious cycle to promote the development of thrombosis and inflammation: platelets mediate neutrophil activation and NETosis, then NETs and granular proteins released from activated neutrophils are retained to the vessel wall by directly interacting with VWF and exert prothrombotic and proinflammatory effects, inducing the release of VWF multimers and promoting platelet adhesion in turn. 2) Both NETs and VWF modify fibrin networks enhancing their procoagulant activity and the resistance of fibrinolysis providing insight into potential therapeutic targets of rt-PA resistance thrombi. 3) Granular components released from activated neutrophils and NETosis not only inactivate ADAMTS13 via chemical modification or competitive combination but also cleave VWF. 4) ADAMTS13 binds and cleaves VWF multimers to prevent excessive platelet aggregation. 5) Neutrophils extravagates into the intima with the help of VWF-platelet complex. VWF, von Willebrand Factor; ADAMTS13, a disintegrin-like and metalloproteinase with thrombospondin type-1 motifs, member 13.