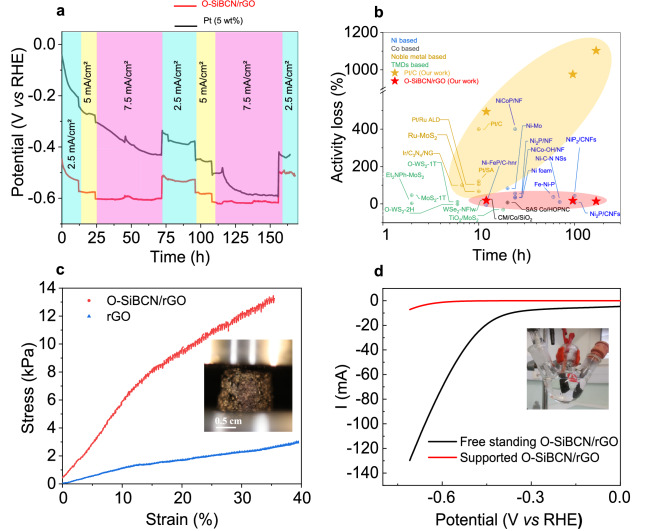
Figure 7.
(a) Chronopotentiometry measurements for supported Pt/C electrodes and O-SiBCN/rGO composite over 168 h. (b) Change in overpotential (%) for O-SiBCN/rGO and other electrocatalysts in the literature: (Ni-FeP/C-hnr62 (hnr = hallow nanorod), Ni2P/CNFs63 (CNFs = carbon nanofibers), NiP2/CNFs63, Ni foam64, NiCo-OH/NF64 (NF = nickel foam), Ni2P/NF64, NiCoP/NF64, Ni-C-N NSs65 (NSs = nanosheets), Ni-Mo66, Fe–Ni-P67, CM/Co/SiO268 (CM = carbon material), SAS/Co/HOPNC69 (SAS = single-atomic Co sites, HOPNC = embedded in hierarchically ordered porous N-doped carbon), Ir/C3N4/NG70, Pt/Ru ALD71 (ALD = atomic layer deposition), Pt/SA71 (SA = single atomic), Pt/C71, Ru-MoS272, MoS2 1T73, Et2NPh fct MoS273, TiO2/MoS274, O-WS2-1T75, O-WS2-2H75, WSe2 NFlw76 (NFlw = nano flowers). (c) Compression curves of the rGO foam and the O-SiBCN/rGO composite. The inset shows that the composite remains stable after compression. (d) Comparison of the polarization curves from the O-SiBCN/rGO composite catalyst deposited on the glassy carbon electrode and free-standing O-SiBCN/rGO. Note that the potentials of the free-standing electrode were not iR loss corrected.