Figure 3.
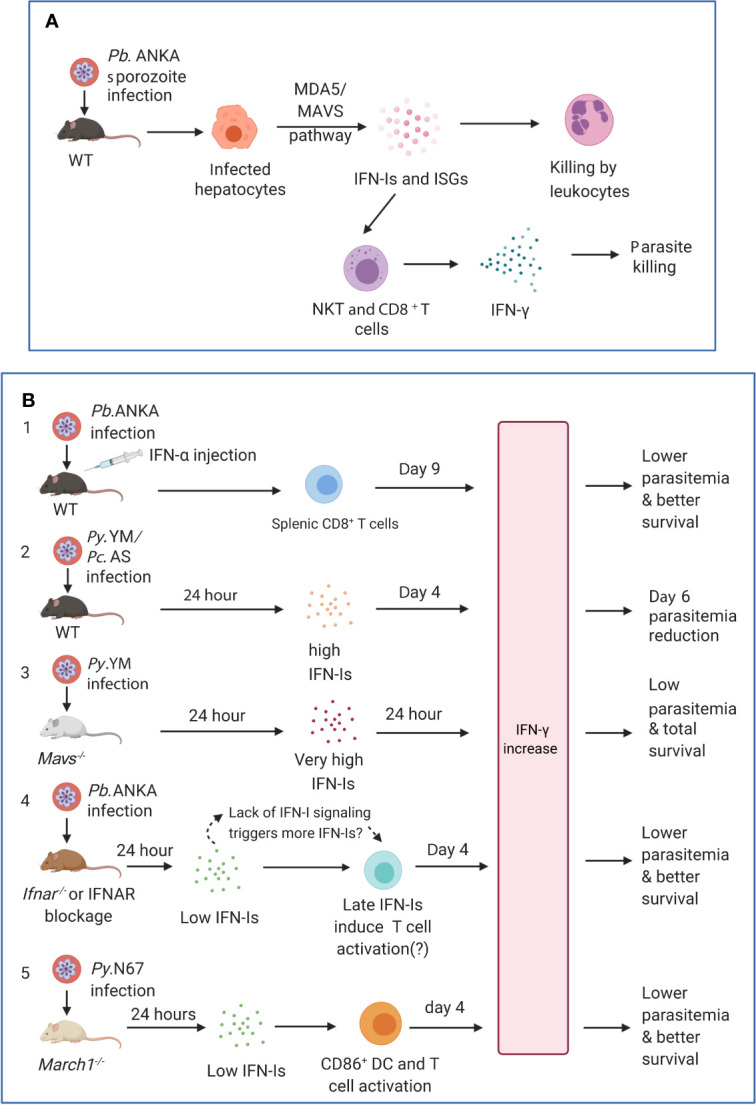
Timing and levels of interferon production may determine the outcomes of malaria parasite infections. (A) The protection against liver stages relies on the activation of innate immunity involving IFN-I responses and immune cell infiltration. Infection of C57BL/6 mice with P. berghei ANKA liver-stages induces an IFN-I response through MDA5 signaling pathway in hepatocytes. Infiltrating leukocytes (macrophages and neutrophils) are mobilized to the vicinity of infected hepatocytes by IFN-I signaling (Liehl et al., 2014). IFN-γ–secreting immune cells, in particular CD1d-restricted NKT cells, are also likely the main players responsible for the innate elimination during liver stage (Miller et al., 2014). (B) Several models show that IFN-Is likely function through regulating IFN-γ production, T cell activation, and adaptive immune response to influence parasitemia and disease severity during blood stage infections. First, several studies showed that injections of IFN-α and/or IFN-β were protective ( Supplementary Table 1 ). C57BL/6 mice infected with P. berghei ANKA and injected with IFN-α had significantly (P <0.05) higher levels of IFN-γ (772 ± 73 pg/ml, day 9 pi) than those receiving diluent (180 ± 14 pg/ml), reduced parasitemia, and better host survival (Vigario et al., 2007). Second, C57BL/6 mice infected with P. y. nigeriensis N67 or P. chabaudi AS had elevated levels of IFN-α (~320 and ~450 pg/ml, respectively) 24 h pi (Kim et al., 2012; Wu et al., 2020). Mavs−/− mice infected with P. y. nigeriensis N67 or Ifnar−/− mice infected P. chabaudi AS had increased day 6 parasitemia (parasitemia increases in Ifnar−/− mice may not be significant but had reduced ability to resolve parasitemia later) (Voisine et al., 2010; Kim et al., 2012; Wu et al., 2014). IFN-Is appear to work with IFN-γ in controlling parasitemia during early infection. Compared with P. y. yoelii YM infected WT C57BL/6 mice that produced very low IFN-Is 24 h pi, P. y. nigeriensis N67-infected mice had significantly higher IFN-α/β, IFN-γ and IL-6 24 h pi (Wu et al., 2020). Additionally, Ifnar1−/−Ifngr1−/− mice infected with P. chabaudi AS exhibited higher mortality than WT or Ifnar1−/− mice and were not able to completely clear parasites (Kim et al., 2012). Third, Mavs−/− mice infected with P. y. yoelii YM produced very high levels of IFN-α (~2,800 pg/ml), IFN-β (~2,000 pg/ml) and IFN-γ (>1,200 pg/ml) 24 h pi and all survived the infections (Yu et al., 2016). All these models suggested early production (24 h) of IFN-α/β and IFN-γ can help control parasitemia and may improve host survival. Fourth, in P. berghei ANKA-infected WT C57BL/6 mice, low levels of IFN-α/β were observed 24 h after pi (Haque et al., 2011; Haque et al., 2014). Depletion or blockage of IFN-I signaling using Ifnar−/− mice or anti-IFNAR antibody treatment results in higher levels of IFN-γ, better parasite control, and improved host survival (Haque et al., 2011; Haque et al., 2014). Interestingly, higher levels of IFN-α were observed in WT mice 48 h pi (~70 pg/ml IFN-α vs ~20 pg/ml at 24 h) and day 4 pi (~200 mg/ml IFN-α), suggesting that blockage of IFN-I signaling may stimulate IFN-I response. Unfortunately, the IFN-I levels in Ifnar−/− mice or mice treated with anti-IFNAR were not measured at additional time points. Similarly, anti-IFNAR antibody treatment of C57BL/6 mice infected with P. y. yoelii 17XNL significantly reduced days 16 and 21 parasitemia through inhibition of T regulatory 1 response, enhancement of Tfh cell accumulation and better humoral immunity (Zander et al., 2016). Again, the levels of IFN-Is in the anti-IFNAR treated animals were not reported. Serum levels of IFN-γ between anti-IFNAR antibody treated and non-treated mice were similar at day 16 pi when parasitemia began to show significant difference. It is possible that the lack of IFN-I signaling in these models prompts the system to produce more IFN-Is, and that IFN-Is and IFN-γ work together through regulating immune cell populations and antibody production to control the infections. Fifth, March1−/− mice infected with P. y. nigeriensis N67 (or P. y. yoelii YM) had low levels of IFN-Is 24 h pi but had significantly increased IFN-γ and IL-10 day 4 pi due to decreased degradation of CD86/MHCII and T cell activation, leading to reduced parasitemia and better host survival (Wu et al., 2020). IFN-γ was shown to be a key player in controlling parasitemia and host survival. These observations suggest key roles of early IFN-Is (24 h pi) and IFN-γ in later stages of infection (day 4 or later) and emphasize the importance of measuring IFN-Is, IFN-γ, and other cytokines during the course of blood infection for better understanding of protection mechanisms mediated by IFNs. Pb.ANKA, P. berghei ANKA; Py.N67, P. y. nigeriensis N67; Py.YM, P. y. yoelii YM; Pc.AS, P. chabaudi AS.
