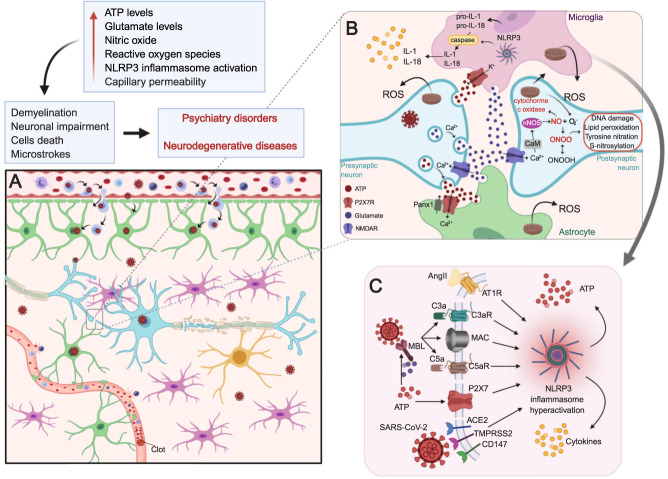
Fig. 2. P2X7 receptor-mediated neuroinflammatory implications of SARS-COV2 invasion in the CNS.
A SARS-CoV-2 may alter brain function by reaching the central nervous system (described in Fig. 1) and/or through the cytokine storm-mediated effects. The result is a neuroinflammatory process characterized by microglia (pink) hyperactivation, astrocyte (green) stimulation, and demyelination (yellow caps) of neurons (blue). In addition, the cytokine storm induces blood-clot formation and increased capillary (red) permeability resulting in embolic and hemorrhagic strokes, respectively. B In a molecular view, the distressed cells release pro-inflammatory cytokines (yellow circles) and ATP (red circles). ATP activates P2X7 receptors (red) expressed mainly in microglia (pink) and astrocytes (green) resulting in increased Ca2+ influx and glutamate (purple circles) release. Glutamate activates NMDA receptors expressed in nerve terminals (blue), which enable Ca2+-dependent exocytosis of ATP and more glutamate release. In this way, an auto regenerative loop is formed causing a massive release of these neurotransmitters augmenting excitotoxicity and cell death. In the postsynaptic neuron (blue), increased [Ca2+]i leads to Ca2+-calmodulin (CaM) complex formation and consequent nNOS activation. NO production mediates neurotoxicity via several mechanisms. NO interacts with the iron–sulfur centers in the mitochondrial electron transport chain impairing cellular energy production. NO also produces reactive nitrogen species and reactive oxygen species (ROS). Reaction of NO and superoxide ion (O2−, formed by nNOS under low arginine concentrations) generates peroxynitrite (ONOO-) and peroxynitrous acid (ONOOH). These free radicals can also decompose into other reactive species, such as hydroxyl radical and peroxides. Oxidative stress from free radicals includes DNA damage, lipid peroxidation, tyrosine nitration, and excess S-nitrosylation. These structural changes can lead to protein misfolding and aggregation causing neuronal impairment and/or death. In microglia, K+ efflux mediated by P2X7 receptor activation may trigger NLRP3 inflammasome assembly and activation through NIMA-related serine/threonine kinase 7 (Nek7) binding. NLRP3 inflammasome mediates the activation of caspase-1, which induces the maturation of interleukins (IL) by cleaving pro-IL-1β and pro-IL-18 in IL-1β and IL-18, respectively. The mature forms of cytokines are secreted worsening the neuroinflammatory process established. C The hyperactivation of NPLR3 inflammasome and consequent release of cytokines and ATP can occur by different routes. (I) Activation of the renin–angiotensin system (RAS) leads to elevated levels of angiotensin II (Ang II) that binds to the AT1R receptor. (II) The N proteins of the SARS-CoV-2 virus activate ComC in a mannan binding lectin (MBL)-dependent manner, producing C3a and C5a anaphylatoxins and forming the non-lytic C5b/C9 membrane attack complex (MAC). ATP can also activate MBL and induce this response. (III) P2X7 receptor activation by ATP induces K+ influx and inflammasome activation. (IV) SARS-CoV-2 invasion through ACE2, TMPRSS2 or CD147 activates the inflammasome in target cells. Hyperactivation of these pathways leads to activation of caspase 1, release of mature IL-1β and IL-18, the insertion of gasdermin D channels in the cell membrane and the release of danger-associated molecular pattern molecules (DAMPs), which amplify the innate immune response and may lead to cell death by pyroptosis. Created with BioRender.com.