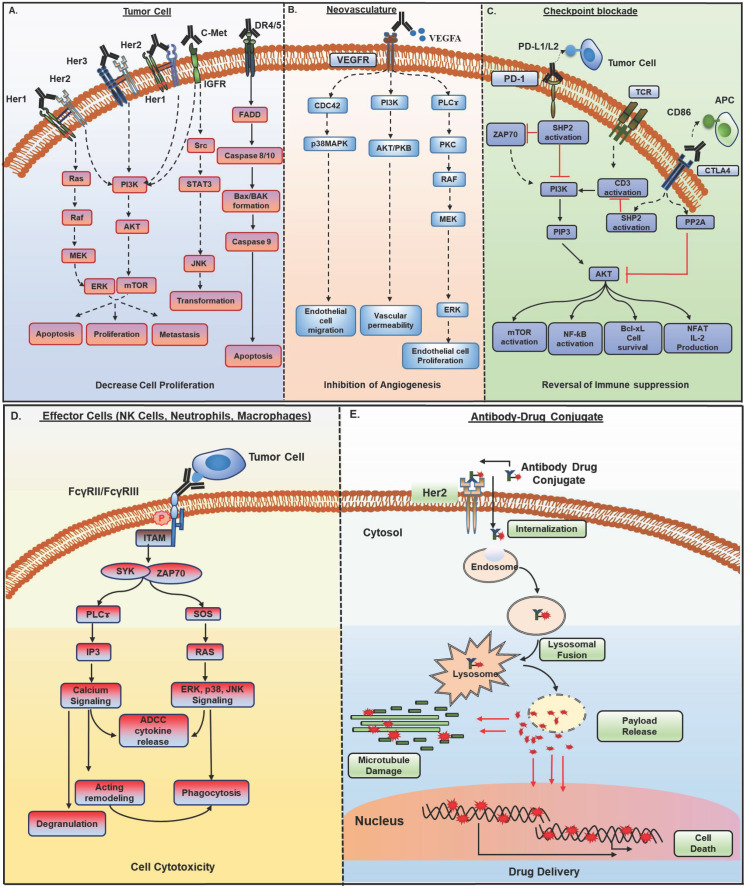
Figure 1.
Mechanisms of action of monoclonal antibodies (mAb) in cancer therapy. Antibody-based therapeutic strategies (A-E) in solid tumors include both direct and indirect tumor cell killing. A) Antibodies acting by functional neutralization of receptor(s) on tumor cells. Antibody binding to overexpressed HER family receptors (HER1, HER2, HER3) on tumor cells, interfere with ligand binding or inhibit their homo- and hetero- dimerization with other HER family members and inhibit activation of downstream MAPK/ERK and PI3K/AKT signaling pathways that promote growth, migration, and proliferation of tumor cells. Antibodies against c- met receptor block STAT3/JNK and PI3K/AKT signaling and inhibit tumor cell transformation, survival, and proliferation. Recently developed class of agonistic antibodies to TNF superfamily death receptors DR4 and DR5 stimulate apoptosis through Bax/Bak and Caspase 9 pathway. B) Binding of antibodies to tumor vasculature receptors VEGFR1, VEGFR2, or their ligand VEGFA inhibits endothelial cell proliferation, migration, vascular permeability, and angiogenesis by interfering with PI3K/AKT, MAPK, and MEK/ERK signaling. C) Antibodies to immune checkpoint molecules, which include inhibitory (CTLA4) and co-inhibitory receptors (PD-1) present on immune cells or their ligands upregulated by tumor cells (PD-L1), reverse T cell exhaustion. Anti-PD-1 antibodies interfere with tyrosine phosphatase SHP2 recruitment and allow TCR (CD3) induced PI3K/AKT and MAPK signaling activation, cell survival, and proliferation. Anti-CTLA4 antibodies inhibit PP2A recruitment and CD3 dephosphorylation and activate PI3K/AKT, mTOR, NF-kB signaling pathways. D) Antibodies bound to tumor cells display antibody-dependent cell cytotoxic (ADCC) activity by engaging FcγR, present on the effector cells such as NK cells, neutrophils, and macrophages. This interaction induces ITAM phosphorylation and binding to tyrosine kinases ZAP-70 and SYK, which in turn activate PI3K and SOS. PIP3 generated through PI3K activation recruit BTK and PLCγ. RAS, BTK, and PLC activate downstream ERK, p38 and JNK signaling pathway along with calcium release from the endoplasmic reticulum (ER), which result in the release of cytokines and cytotoxic granules as IFNγ, perforin, and granzymes from NK cells and actin remodeling, which finally cause tumor cell apoptosis. Antibody bound tumor cells are recognized by neutrophils and macrophages and trigger oxidative burst and phagocytosis by neutrophils and macrophages, leading to lysosomal degradation of tumor cells. E) Antibody-drug conjugates (ADCs) possess specificity of mAb and cytotoxic potential of payload drug. ADCs bind to target antigen, get internalized, and undergo endocytic processing. Once in the cell, ADCs cleavage occurs, and the active cytotoxic drug is released into the cytoplasm where it inhibits microtubule polymerization and subsequently, tumor cell death. HER, human epidermal growth factor receptor; MAPK, mitogen- activated protein kinase; ERK, extracellular signal- regulated kinase; MEK, MAPK/Erk kinase 1/2; PI3K, phosphoinositide 3-kinase; SOS, son of sevenless homologue; STAT3, signal transducer and activator of transcription 3; JNK, c-JUN N- terminal kinase; DR4, death receptor 4; DR5, death receptor 5; Bax, Bcl-2-associated X protein; VEGFR1, vascular endothelial growth factor receptor-1; VEGFR2, vascular endothelial growth factor receptor-2; VEGFA, vascular endothelial growth factor A; FcγR; Fc gamma receptor; PIP3, phosphatidylinositol-3,4,5-trisphosphate; NK, natural killer; ITAM, immunoreceptor tyrosine- based activation motif; SYK, spleen tyrosine kinase; BTK, bruton's tyrosine kinase; PLCγ, phospholipase C gamma; RAS, rat sarcoma viral oncogene homolog; CTLA-4, cytotoxic T lymphocyte antigen 4; PD-1, program death-1; PD-L1, program death-1 ligand; SHP2, src homology phosphatase 2; TCR, t cell receptor; CD3, cluster of differentiation 3; mTOR, mammalian target of rapamycin; NF-kB, nuclear factor kappa-light-chain-enhancer of activated B cells.