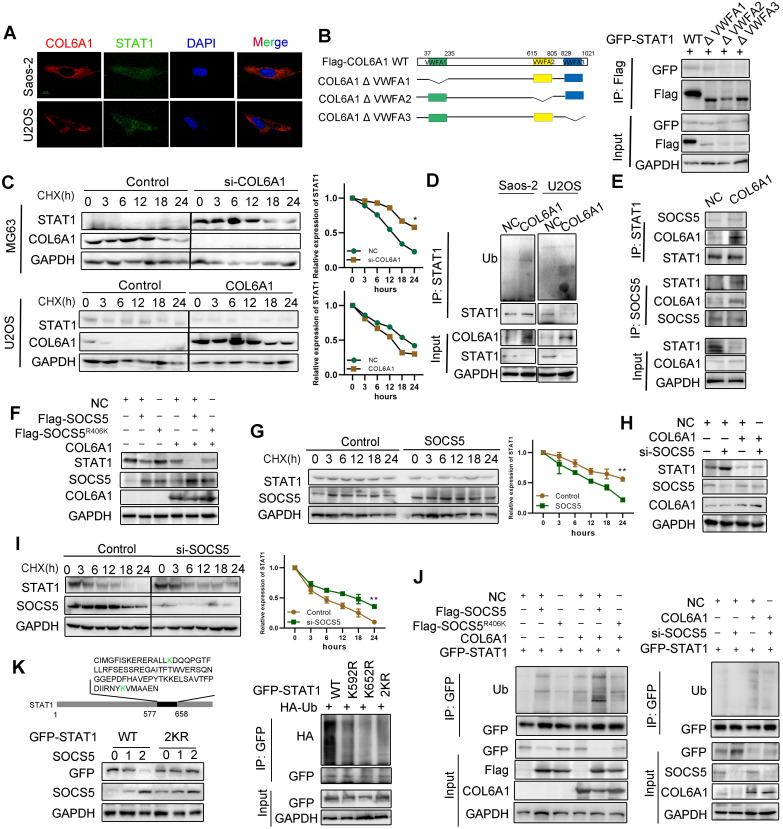
Figure 5.
COL6A1 interacted with E3 ligase SOCS5 to promote STAT1 degradation. A. Co-localization of COL6A1 and STAT1 in ESCC cells were examined by using confocal microscopy (scale bar, 10 µm). B. The interaction of GFP-STAT1 and Flag-COL6A1 wide type, VWFA1, VWFA2, VWFA3 domains deletion mutations were detected by co-immunoprecipitation in 293T cells. C. COL6A1 was transfected into the OS cells in the presence of cycloheximide (CHX, 200 µg/mL) for indicated times. Cell lysates were immunoblotted by antibodies as indicated. The data were quantified using Image J software. D. STAT1 ubiquitination was detected by immunoprecipitation with anti-STAT1 antibody and immunoblotting with an anti-Ub antibody. E. The interaction of STAT1, COL6A1 and SOCS5 was detected by co-immunoprecipitation in U2OS. F. SOCS5 decreased STAT1 protein. U2OS cells were transfected with Flag-SOCS5 or Flag-SOCS5R406K as well as control or COL6A1 transfection. The protein expression level of STAT1 was assayed by western blot. G. The cells expressing wide type SOCS5 were treated with CHX. The protein levels of STAT1 and SOCS5 were analyzed by western blot. H. Knockdown SOCS5 increased STAT1 protein. U2OS cells were transfected with si-SOCS5 as well as control or COL6A1 transfection. I. U2OS cells were transfected with control or SOCS5 siRNAs treated with CHX (200 µg/mL), the protein levels of STAT1 and SOCS5 were analyzed by western blot. J. SOCS5 ubiquitylates STAT1. U2OS cells were transfected with indicated plasmids or siRNA for 48 h. Cell lysates were immunoprecipitated with anti-GFP and analyzed by immunoblotting with indicated antibodies. K. U2OS cells were transfected with indicated plasmids. lysates were immunoprecipitated with anti-GFP, and western blots were performed to analyze the presence of indicated proteins and levels of ubiquitination. Data represent the mean ± SD of 3 separate determinations. *p < 0.05, **p < 0.01, ***p < 0.001 by Student's t test.