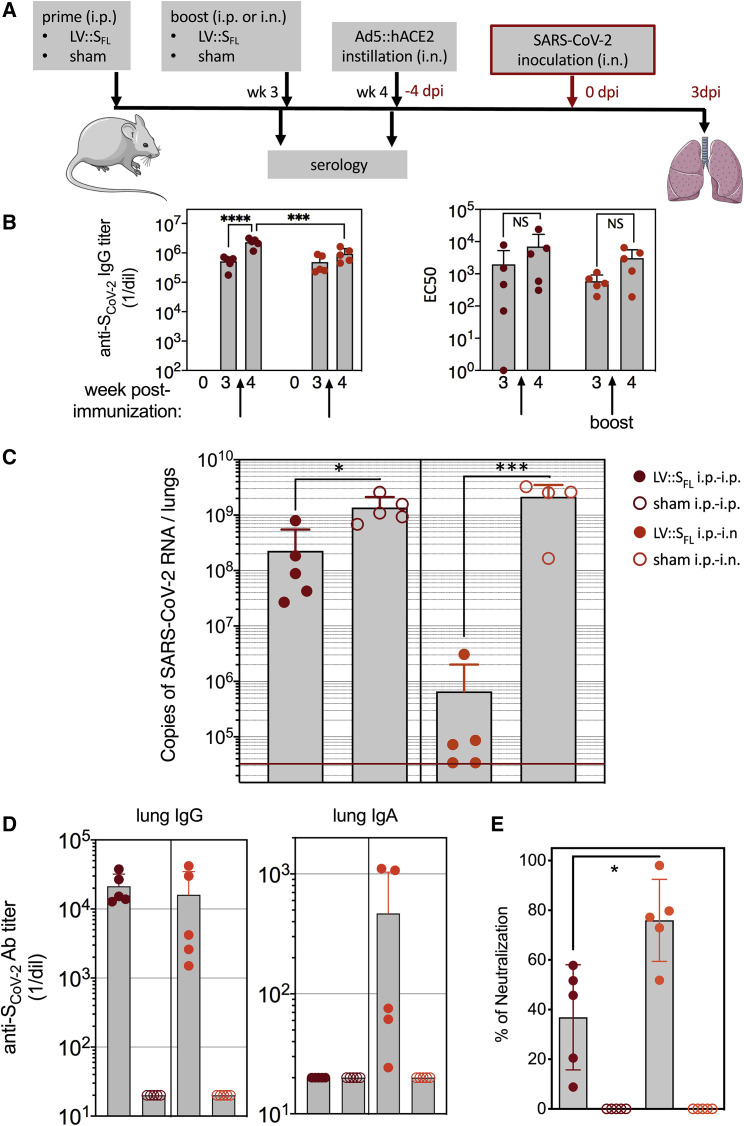
Figure 4.
Intranasal boost with LV::SFL strongly protects against SARS-CoV-2 in mice
(A) Timeline of the LV-based prime-boost strategy, followed by Ad5::hACE2 pretreatment and SARS-CoV-2 challenge.
(B) Titers of anti-SCoV-2 IgG as quantitated by ELISA in the sera of C57BL/6 mice primed at week 0 via the i.p. route and boosted at week 3 via the i.p. or i.n. route (left). Titers were determined as a mean endpoint dilution before the boost (week 3) and challenge (week 4). ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; two-way ANOVA followed by Sidak’s multiple-comparison test. NS, not significant. The neutralization capacity of these sera is indicated as EC50 (right). See also Figure S4A.
(C). Lung viral loads at 3 dpi in mice primed (i.p. route) and boosted (i.p. or i.n. route) with LV::SFL. Sham-vaccinated mice received an empty LV. The red line indicates the detection limit. Statistical significance of the differences in the viral loads was evaluated by a two-tailed unpaired t test; ∗p < 0.0139, ∗∗∗p < 0.0088.
(D) Titers of anti-SCoV-2 IgG and IgA Abs determined in the clarified lung homogenates by ELISA with foldon-trimerized SCoV-2 for coating. See also Figure S4B.
(E) Neutralizing activity of the clarified lung homogenates as determined for 1/5 dilution. Statistical significance of the difference was evaluated by a Mann-Whitney U test (∗p < 0.0159). See also Figure S3.